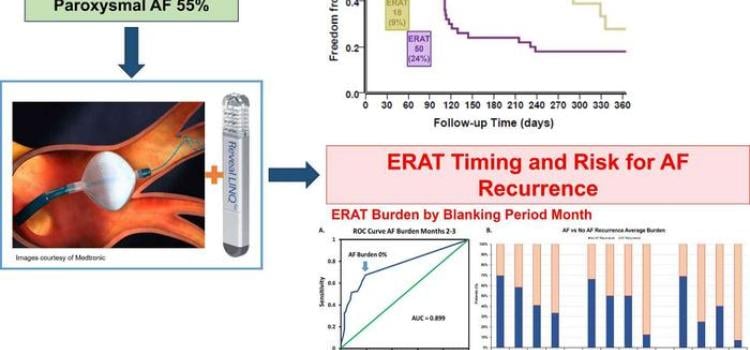
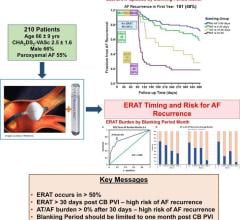
April 18, 2024 — New evidence-based research calls into question the conventional three-month blanking period immediately after atrial fibrillation (AF) ablation, when early occurrences of AF are ...
April 18, 2024 — New evidence-based research calls into question the conventional three-month blanking period ...
April 18, 2024 — Karoo Health, the only operational provider of cardiac value-based care (VBC) enablement with published ...
April 18, 2024 — Bayer AG and Asklepios BioPharmaceutical, Inc., a gene therapy company wholly owned and independently ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 17, 2024 — Getinge and Cook Medical announced an exclusive sales and distribution agreement for the iCast covered ...
April 17, 2024 —CPR Therapeutics, Inc. (CPR-T), an early-stage medtech startup funded by the N.I.H and N.S.F to develop ...
April 16, 2024 — Each year more than 500,000 Americans undergo percutaneous coronary intervention, or PCI, a minimally ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 16, 2024 — Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel ...
April 16, 2024 — CVRx, Inc., a commercial-stage medical device company, announced today the availability of additional ...
April 15, 2024 — The U.S. Food and Drug Administration (FDA) announced Abbott/Thoratec Corp. is recalling HeartMate II ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
April 12, 2024 — Simpson Interventions, Inc., a pioneering medical technology company specializing in cardiovascular ...
April 12, 2024 — HeartBeam, Inc., a medical technology company focused on transforming cardiac care through the power of ...
April 12, 2024 — University of Virginia School of Medicine researchers have discovered a gene on the Y chromosome that ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
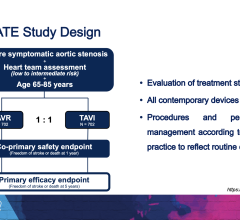
April 11, 2024 — Transcatheter aortic valve replacement (TAVR) was found to bring no increased risks and was associated ...
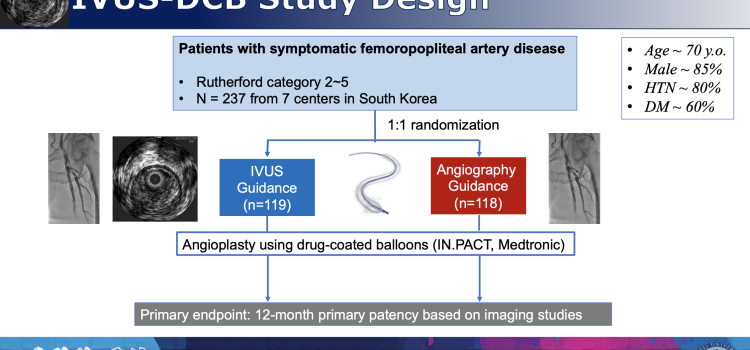
April 11, 2024 — One-year success rates from angioplasty procedures to open clogged arteries in the legs were ...
April 11, 2024 — People with a buildup of fatty atherosclerotic plaque in the heart’s arteries considered at risk of ...



 April 18, 2024
April 18, 2024