April 15, 2024 — The U.S. Food and Drug Administration (FDA) announced Abbott/Thoratec Corp. is recalling HeartMate II and HeartMate 3 Left Ventricular Assist System (LVAS) due to an issue called ...
April 16, 2024 — Each year more than 500,000 Americans undergo percutaneous coronary intervention, or PCI, a minimally ...
April 16, 2024 — Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel ...
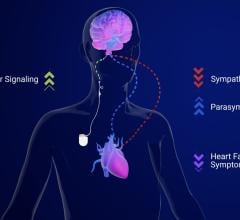
April 16, 2024 — CVRx, Inc., a commercial-stage medical device company, announced today the availability of additional ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
April 15, 2024 — The U.S. Food and Drug Administration (FDA) announced Abbott/Thoratec Corp. is recalling HeartMate II ...
April 12, 2024 — Simpson Interventions, Inc., a pioneering medical technology company specializing in cardiovascular ...
April 12, 2024 — HeartBeam, Inc., a medical technology company focused on transforming cardiac care through the power of ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 12, 2024 — University of Virginia School of Medicine researchers have discovered a gene on the Y chromosome that ...
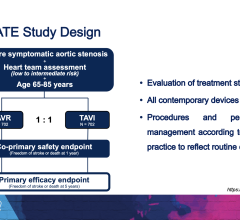
April 11, 2024 — Transcatheter aortic valve replacement (TAVR) was found to bring no increased risks and was associated ...
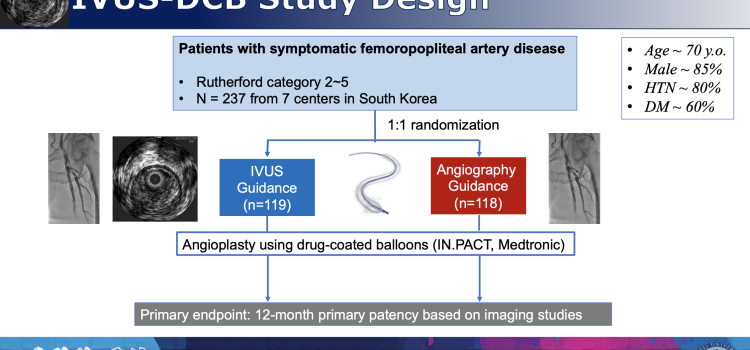
April 11, 2024 — One-year success rates from angioplasty procedures to open clogged arteries in the legs were ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
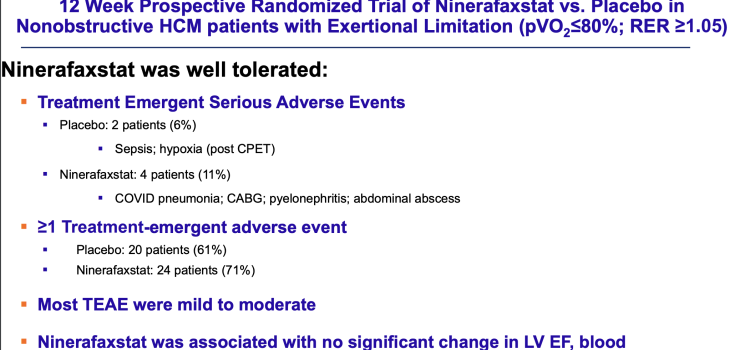
April 11, 2024 — People with a buildup of fatty atherosclerotic plaque in the heart’s arteries considered at risk of ...
April 10, 2024 — The American Medical Association (AMA) released informal survey findings (PDF) showing the ongoing ...
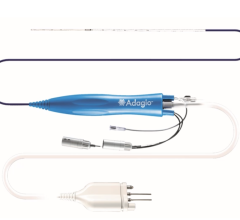
April 10, 2024 — A new technology using ultralow temperature cryoablation (ULTC) has eliminated clinical ventricular ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
April 10, 2024 — An international consensus statement on how to treat atrial fibrillation with catheter or surgical ...
April 9, 2024 — Patients who took an angiotensin-converting enzyme (ACE) inhibitor while undergoing cancer treatment ...
April 9, 2024 — Cathie Biga, MSN, FACC, today became president of the American College of Cardiology and made history as ...



 April 16, 2024
April 16, 2024