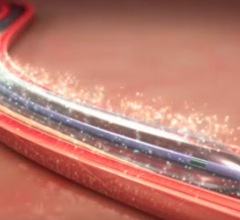
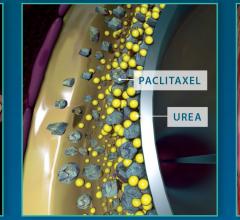
Clinical study data makes the world go around in cardiology and is the basis of setting guidelines in evidence-based ...
Balloon Catheter
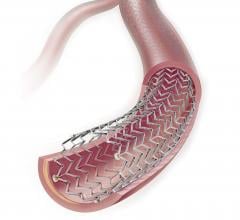
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
January 28, 2019 — Philips announced the latest pooled analysis of patient-level data of over 2,300 patients treated ...
January 17, 2019 — The U.S. Food and Drug Administration (FDA) issued a letter Jan. 17, 2019, to healthcare providers ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
November 30, 2018 — Johnson & Johnson Medical Devices Companies announced that Biosense Webster Inc. has enrolled and ...
In 2009, the GuideLiner Catheter revolutionized the concept of guide extension, creating new possibilities in ...
September 25, 2018 — Orchestra BioMed Inc. announced the three-year clinical results from its Sirolimus Angioplasty ...
September 24, 2018 — New data announced at the 2018 Transcatheter Cardiovascular Therapeutics (TCT) conference, Sept. 21 ...
August 23, 2018 — W. L. Gore & Associates Inc. (Gore) announced U.S. Food and Drug Administration (FDA) 510(k) clearance ...
August 14, 2018 — NuCryo Vascular announced that the company has signed a commercialization agreement with Lokai Medical ...
June 15, 2018 — Medtronic plc has received U.S. Food and Drug Administration (FDA) approval for 200mm and 250mm lengths ...
May 29, 2018 – M.A. MedAlliance SA has raised $37 million to help develop and commercialize the first sirolimus micro ...
May 3, 2018 — The U.S. Food and Drug Administration (FDA) has expanded the indication for the Medtronic In.Pact Admiral ...
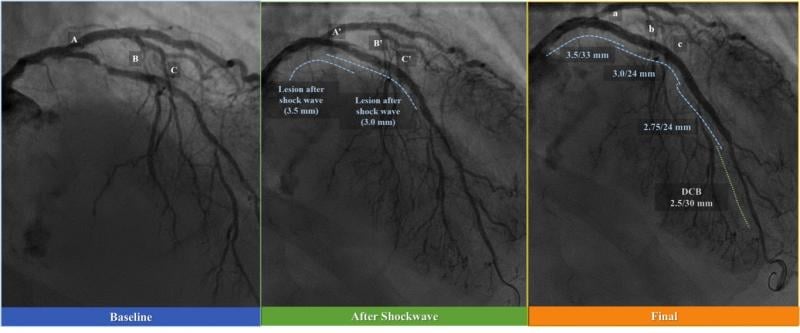
Over the last decade, there have been considerable developments in procedural techniques and technology facilitating the ...
March 21, 2018 — Cardiovascular Systems Inc. (CSI) recently announced that the U.S. Food and Drug Administration (FDA) ...
February 7, 2018 — Medtronic plc recently added to its body of clinical evidence supporting the In.Pact Admiral drug ...

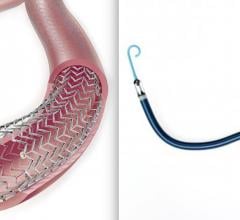
 February 20, 2019
February 20, 2019