January 11, 2024 — It is with great sadness that the Diagnostic and Interventional Cardiology (DAIC) team has learned of the passing of Bruce Wilkoff, MD, FHRS, Director of the Cardiac Pacing and ...
Leads Implantable Devices
News and new technology innovations for leads implantable devices can be found on this channel.
February 12, 2023 — A team at Allina Health Minneapolis Heart Institute at Abbott Northwestern Hospital has successfully ...
January 11, 2024 — It is with great sadness that the Diagnostic and Interventional Cardiology (DAIC) team has learned of ...
July 3, 2023 — Nearly one-third of patients with an implanted device to prevent sudden death have anxiety in the first ...
February 24, 2023 — Biotronik announced that it has received CE approval for the Selectra 3D implant tools to include ...
January 3, 2023 — The market has been studied for the below mentioned-segmentation and regional analysis for North ...
November 22, 2022 — CroíValve has announced the successful First in Human implants of its DUO Tricuspid Coaptation Valve ...
November 2, 2022 — For decades, left ventricular-assist devices (LVADs) have extended the lives of people whose hearts ...
August 5, 2022 — Black people and women with severe heart failure who might be good candidates for surgery to implant a ...

The current standard of care for heart failure (HF) is guideline-directed medical therapy combined with device-based ...
June 16, 2020 - Biotronik has today announced its commitment to giving physicians additional tools to pace in the His ...
September 20, 2019 — BioTrace Medical Inc. announced the company’s key activities at the 31st annual Transcatheter ...
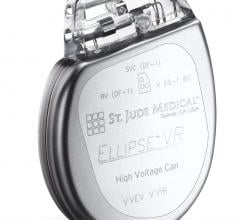
August 5, 2019 — Abbott is recalling the Ellipse Implantable Cardioverter Defibrillators (ICDs) because electrical ...
May 13, 2019 — BioTrace Medical Inc. announced the company’s Tempo Lead has obtained CE Mark certification in Europe for ...
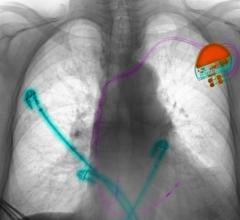
May 6, 2019 — Medtronic has received U.S. Food and Drug Administration (FDA) approval for the Attain Stability Quad MRI ...
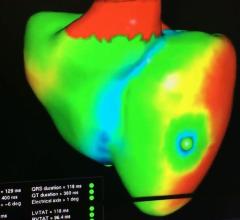
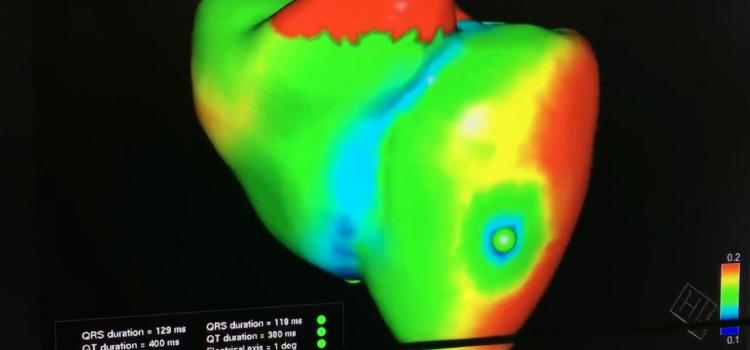
This is a virtual heart with the same electrophysiology characteristics as the real patient being developed to help ...




 February 12, 2024
February 12, 2024