To extract or abandon broken or infected implantable, venous electrophysiology (EP) device leads has been a debate for ...
Cardiac Resynchronization Therapy Devices (CRT)
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.
May 22, 2018 — Medtronic plc announced study results showing its AdaptivCRT algorithm is associated with improved ...
May 18, 2018 — Data on the effectiveness of cardiac resynchronization therapy (CRT) in patients with non-left bundle ...
May 18, 2018 — In a new study, cardiac contractility modulation (CCM) therapy was confirmed to significantly improve ...
Here is an aggregation of all the news and late-breaking studies presented at the 2018 Heart Rhythm Society (HRS) Scient ...
April 30, 2018 — LivaNova announced it completed the sale of its cardiac rhythm management (CRM) business to MicroPort ...
April 12, 2018 — Biotronik U.S. and Aziyo announced a strategic agreement allowing Biotronik to distribute Aziyo's ...
February 26, 2018 — The U.S. Food and Drug Administration (FDA) announced that Medtronic is recalling certain implantabl ...
January 3, 2018 — Abbott announced U.S. Food and Drug Administration (FDA) approval for magnetic resonance (MR) ...
November 25, 2017 — Sitting in, or standing close to the charging port of a Tesla electric vehicle did not trigger a ...
October 20, 2017 — Boston Scientific announced new data from the Multisensor Chronic Evaluation in Ambulatory Heart ...
September 27, 2017 — Boston Scientific recently launched the Resonate family of implantable cardioverter defibrillator ...
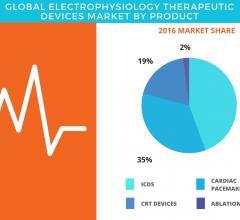
September 11, 2017 — According to the latest market study released by Technavio, the global electrophysiology ...
August 21, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval and commercial availability of ...
July 21, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval and availability of the Intica DX ...


 January 13, 2019
January 13, 2019