January 11, 2024 — It is with great sadness that the Diagnostic and Interventional Cardiology (DAIC) team has learned of the passing of Bruce Wilkoff, MD, FHRS, Director of the Cardiac Pacing and ...
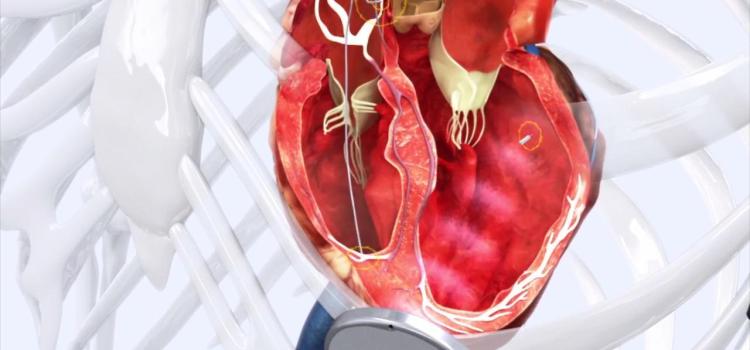
Cardiac Resynchronization Therapy Devices (CRT)
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.
January 11, 2024 — It is with great sadness that the Diagnostic and Interventional Cardiology (DAIC) team has learned of ...
July 18, 2023 — The U.S. Food and Drug Administration (FDA) has issued a Class I recall on the Medtronic ICDs and CRT-Ds ...
May 22, 2023 — Results from a pivotal clinical trial found a leadless pacemaker can deliver cardiac resynchronization ...
Arrhythmias can be described as irregular heart rhythms that are brought on by numerous factors, including heart damage ...
August 19, 2022 — According to a release just issued by the U.S. Food and Drug Administration (FDA), Medtronic is ...
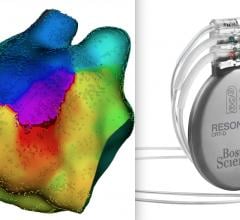
Royal Philips, a global leader in health technology, announced late-breaking results of a large-scale, real-world ...

November 1, 2021 — Here is the list of the most popular content on the Diagnostic and Interventional Cardiology (DAIC) ...
With 1.2 to 1.4 million new electrophysiology (EP) devices being prescribed to patients around the world each year ...
October 27, 2021 — EBR Systems Inc., developer of the world’s first wireless cardiac pacing system for heart failure, ...
October 1, 2021 — The European Society of Cardiology (ESC) guidelines on cardiac pacing and cardiac resynchronization ...
Khaldoun Tarakji, M.D., MPH, associate section head, cardiac electrophysiology, Heart and Vascular Institute at ...
September 13, 2021 — Ablation plus cardiac resynchronization therapy (CRT) is superior to pharmacological rate control ...
August 3, 2021 — In association with Heart Rhythm 2021, Biotronik announced first enrollments in the landmark BIO ...
Samir Saba, M.D., co-director of the University of Pittsburgh Medical Center Heart and Vascular Institute, and chief ...



 January 11, 2024
January 11, 2024