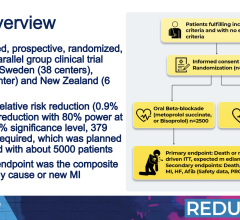
April 8, 2024 — Taking beta blockers after a heart attack did not significantly reduce the risk of death or a second heart attack among people with normal heart pumping ability, as indicated by an ...
Cath Lab
The catheterization lab channel includes content related to interventional technologies for coronary and peripheral artery disease (PAD). Other cath lab transcatheter device technologies covered on this page included percutaneous treatment for stroke, venous interventions, heart valves, hypertension, heart failure and percutenous coronary interventions (PCI).
April 17, 2024 — Getinge and Cook Medical announced an exclusive sales and distribution agreement for the iCast covered ...
April 16, 2024 — Each year more than 500,000 Americans undergo percutaneous coronary intervention, or PCI, a minimally ...
April 16, 2024 — Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel ...
Adjustable parameters on the ACIST® CVI® Contrast Delivery System include, flow rate, volume, pressure limit, rise time ...
April 12, 2024 — Simpson Interventions, Inc., a pioneering medical technology company specializing in cardiovascular ...
April 11, 2024 — One-year success rates from angioplasty procedures to open clogged arteries in the legs were ...
April 8, 2024 — Taking beta blockers after a heart attack did not significantly reduce the risk of death or a second ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
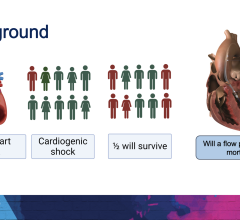
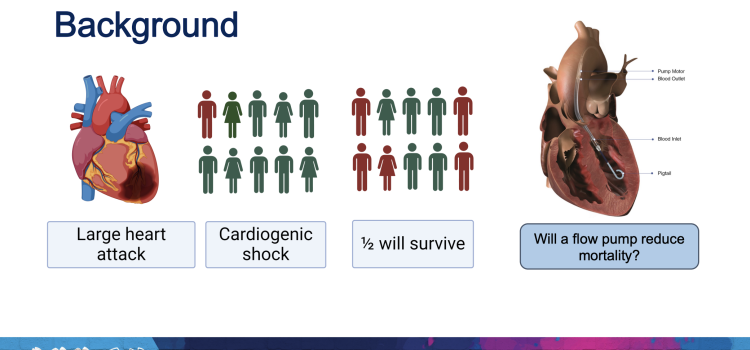
April 8, 2024 — Implantation of the Impella CP micro-axial flow pump in the hours after a heart attack significantly ...
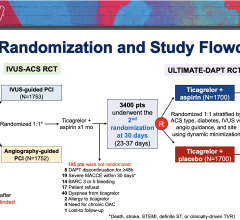
April 8, 2024 — People who have had a heart attack or who are at risk for a heart attack and who stopped taking aspirin ...
April 4, 2024 — The U.S. Food and Drug Administration (FDA) announced that Medos International Sàrl is recalling Cerenov ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...

Here is a Top 10 look at the content that was trending during the month of March on dicardiology.com:
1. CLS Health ...
April 3, 2024 — The U.S. Food and Drug Administration (FDA) announced that Teleflex and Arrow International are ...
April 2, 2024 — Medical device technology developer Concept Medical has announced it has been granted Investigational ...
During cardiology fellowship, my institution had a hand manifold system. I found it difficult to use at times, and it ...

The American College of Cardiology (ACC) has announced key educational and programming highlights for its ACC 73rd ...
March 21, 2024 — Prolocor, Inc., a healthcare startup developing an innovative precision diagnostic test with the goal ...
March 21, 2024 — The U.S. Food and Drug Administration (FDA) announced that Abiomed is recalling the Instructions for ...

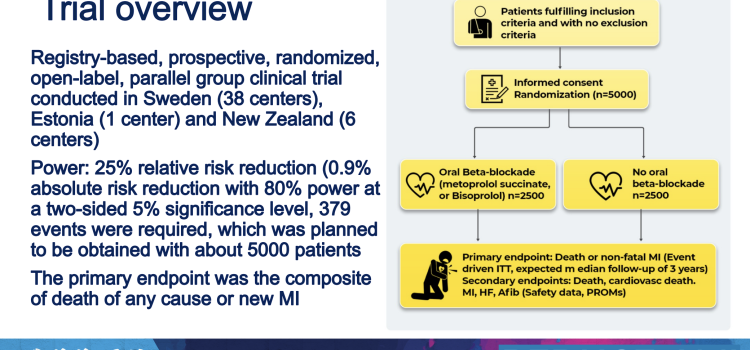



 April 17, 2024
April 17, 2024