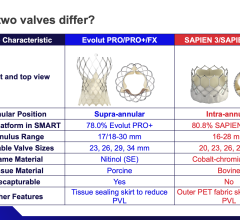
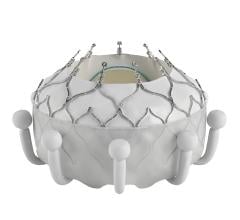
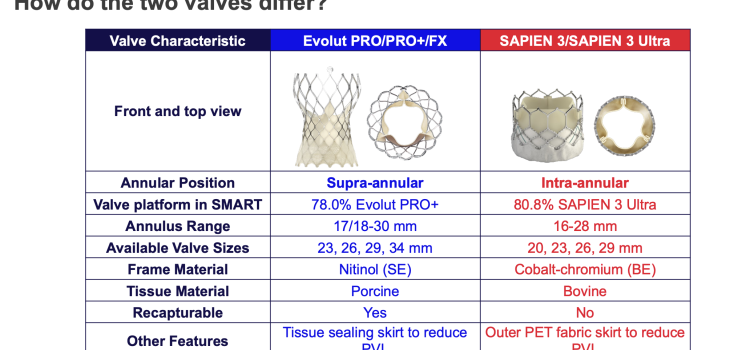
April 23, 2024 — Medtronic plc, a global leader in healthcare technology, today announced the launch of its latest innovation in cardiac surgery, the Avalus Ultra valve. This next-generation surgical ...
Heart Valve Technology
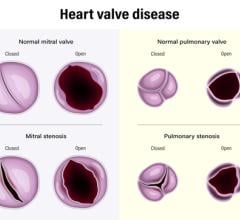
This channel includes news and new device innovations about heart valve technologies, including the aortic valve, mitral valve, pulmonic valve, and tricuspid valve. This includes information on transcatheter valve technologies like transcatheter aortic valve replacement (TAVR, or implantation TAVI), transcatheter mitral valve repair or replacement (TMVR), transcatheter and surgical valve repairs, and surgical replacement valves. Newer devices are now being used for transcatheter tricuspid valve repair replacement (TTVR).
April 23, 2024 — Medtronic plc, a global leader in healthcare technology, today announced the launch of its latest ...
April 17, 2024 —CPR Therapeutics, Inc. (CPR-T), an early-stage medtech startup funded by the N.I.H and N.S.F to develop ...
April 16, 2024 — Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel ...
April 9, 2024 — UC Davis Health cardiology team members are among the first in the country to treat patients with tricus ...
April 9, 2024 — Administering tranexamic acid (TxA), a drug used to reduce bleeding during heart surgery, topically ...
April 9, 2024 — People with a small aortic annulus, a part of the heart’s anatomy where the left ventricle meets the ...
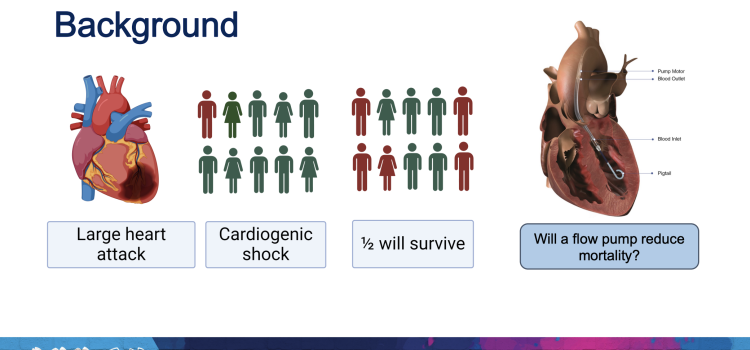
April 8, 2024 — Implantation of the Impella CP micro-axial flow pump in the hours after a heart attack significantly ...
April 2, 2024 — Abbott announced that the U.S. Food and Drug Administration (FDA) approved the company's first-of-its ...
April 1, 2024 — Roughly 25,000 Americans die each year from valvular heart disease, but researchers from Rutgers Health ...
February 26, 2024 — Hackensack Meridian Jersey Shore University Medical Center and Hackensack University Medical Center ...

The DAIC team has learned of the passing of Alain Cribier, MD, FACC, heralded as the man who pioneered the first transca ...
February 2, 2024 — Edwards Lifesciences Corporation announced the company’s EVOQUE tricuspid valve replacement system is ...
You may not know Carol Barr, but in the future, she could save your life.
Barr’s death at 39 from sudden cardiac ...
January 4, 2024 — Laguna Tech USA, a privately-held medical technology company dedicated to innovations in structural ...
January 4, 2024 — Findings from a published case series research letter by the Henry Ford Health Structural Heart ...


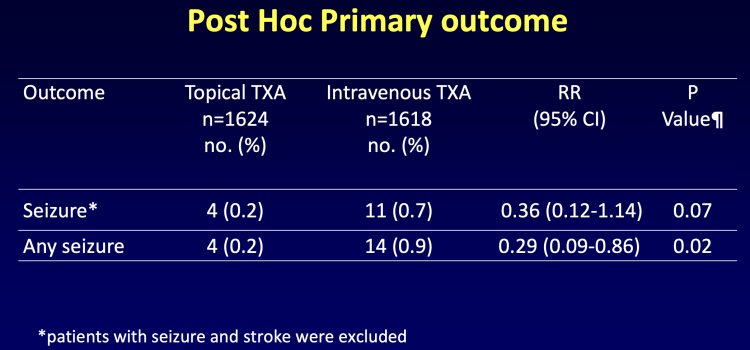

 April 23, 2024
April 23, 2024