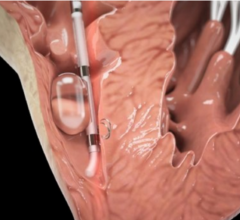
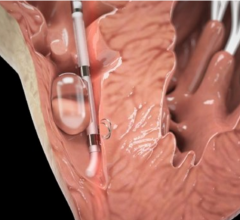
May 9, 2017 — Biotronik announced U.S. Food and Drug Administration (FDA) approval and the launch of Sentus ProMRI, the ...
Leads Implantable Devices
News and new technology innovations for leads implantable devices can be found on this channel.
April 12, 2017 — Biotronik announced the European launch of a new lead for tachycardia therapy that features a helical ...
January 13, 2017 — BioTrace Medical Inc. announced in December the first commercial use of the company’s Tempo Temporary ...
December 19, 2016 — BioTrace Medical Inc. announced the first commercial use of the company’s Tempo Temporary Pacing ...
October 27, 2016 — BioTrace Medical Inc. said it received U.S. Food and Drug Administration (FDA) 510(k) clearance for ...
May 9, 2016 —St. Jude Medical announced presentation of the MultiPoint Pacing (MPP) Investigational device exemption ...
May 9, 2016 – A new, large study shows that 95.7 percent of individual leads were successfully removed using a procedure ...
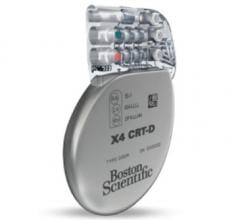
April 28, 2016 — Boston Scientific has received U.S. Food and Drug Administration (FDA) approval for a suite of products ...
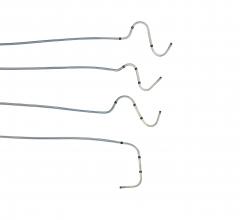
March 29, 2016 — St. Jude Medical Inc. announced CE Mark approval and launch of three new Quartet left ventricular (LV) ...
March 4, 2016 — Boston Scientific has received U.S. Food and Drug Administration (FDA) approval for the Acuity X4 ...
February 17, 2016 — St. Jude Medical Inc. announced U.S. Food and Drug Administration (FDA) approval of the first-to ...
January 26, 2016 — St. Jude Medical Inc. is recalling the Optisure implantable cardioverter defibrillator (ICD) leads ...
May 19, 2015 — A new epicardial pacing lead has been cleared by the U.S. Food and Drug Administration (FDA) as an option ...
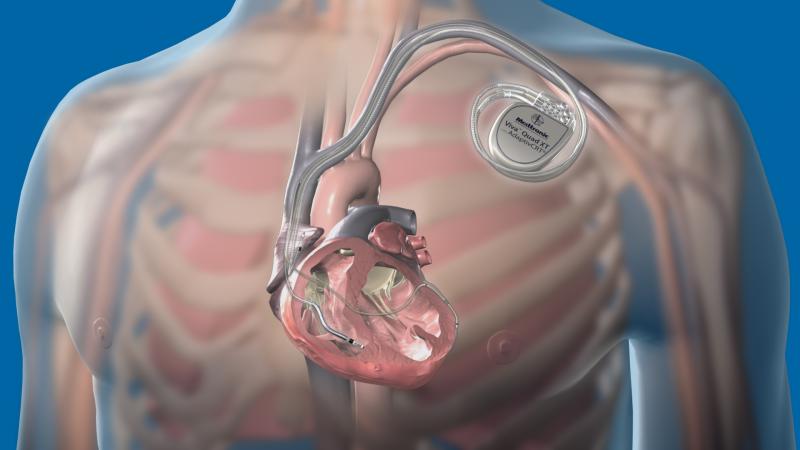
December 17, 2014 – Medtronic Inc. announced the U.S. Food and Drug Administration (FDA) approval and commercial launch ...
December 11, 2014 — A new study evaluating Optim-insulated implantable cardioverter defibrillator (ICD) leads found low ...

 May 09, 2017
May 09, 2017