July 6, 2023 — BIOTRONIK announced U.S. Food and Drug Administration (FDA) approval of its portfolio of Amvia Edge pacemakers and cardiac resynchronization therapy pacemaker (CRT-P), its latest ...
Magnetic Resonance Imaging (MRI)
Cardiac MRI creates images from the resonance of hydrogen atoms when they are polarized to face in one direction and then hit with an electromagnetic pulse to knock them off axis. The wobbling of the atoms is what is recorded by computers and used to reconstruct the images. Cardiac MR allows very detailed visualization of the myocardial tissue above the resolution found with cardiac CT. Using different protocol sequences, various contrast type images can be created with MRI to enhance various tissues or to provide physiological data on the function of the heart. This section includes MR analysis software, MRI scanners, gadolinium contrast agents, and related magnetic resonance accessories.
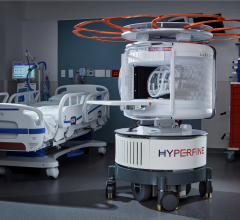
February 21, 2024 — Hyperfine, Inc., a groundbreaking health technology company that has redefined brain imaging with ...
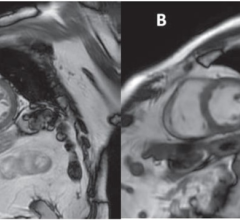
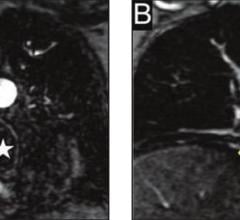
February 12, 2024 — According to the American Journal of Roentgenology (AJR), free-breathing cine-deep learning (DL) may ...
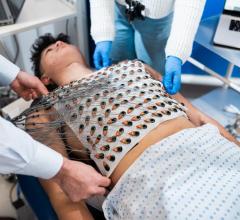
December 19, 2023 — A vest that can map the electrical activity of the heart in fine detail could potentially be used to ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
November 21, 2023 — Heuron, a medical AI (artificial intelligence) imaging software solution company, under the ...
November 17, 2023 — Researchers from the University of Minnesota Medical School examining the cause of cardiomyopathy ...
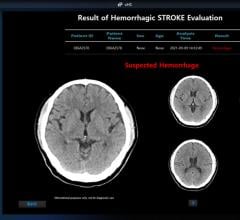
November 3, 2023 — In the U.S., someone has a stroke every 40 seconds and dies from it every three minutes and 14 ...
August 24, 2023 —Royal Philips, a global leader in health technology, highlighted how it integrates AI in cardiac ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
July 11, 2023 — Hyperfine, Inc., the groundbreaking medical device company that created the Swoop system, the world’s ...
June 28, 2023 — Liver disease, the UK’s third leading cause of premature death, poses a significantly greater threat to ...
June 22, 2023 — An estimated 3 million patients visit emergency departments each year with acute chest pain and mildly ...




 February 21, 2024
February 21, 2024