May 15, 2019 — The Heart Rhythm Society (HRS) had 21 late-breaking study presentations at the 2019 Heart Rhythm ...
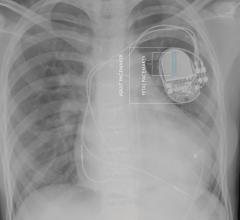
Pacemakers
This channel includes news and new technology innovations for pacemakers used to treat bradycardia.

May 15, 2019 — A new infection risk scoring system has been developed based on data from the large PADIT Trial.[1] The ...
May 14, 2019 – Results from new research show that passengers with cardiac implantable electronic devices (CIEDs), such ...
May 13, 2019 – Results from a new survey are the first to report a large discrepancy in patient’s knowledge of their ...
May 7, 2019 — The U.S. Food and Drug Administration (FDA) issued a safety communication to alert healthcare providers ...
March 29, 2019 — A research team from Imperial College London believes a new software could speed up the diagnosis and ...
Khaldoun Tarakji, M.D., MPH, associate section head, section of electrophysiology and pacing in the Robert and Suzanne ...
March 21, 2019 — The U.S. Food and Drug Administration (FDA) approved Impulse Dynamics’ Optimizer Smart system for ...
February 20, 2019 — Medtronic is recalling its dual chamber implantable pulse generators (IPGs) due to the possibility ...

To extract or abandon broken or infected implantable, venous electrophysiology (EP) device leads has been a debate for ...
October 17, 2018 — The U.S. Food and Drug Administration (FDA) has reviewed information about potential cybersecurity ...
October 2, 2018 — Two-year results of the Moderato I Study demonstrated immediate, substantial and sustained reduction ...
June 28, 2018 — Investigators at Children's Hospital Los Angeles (CHLA) and the University of Southern California (USC) ...
May 24, 2018 — Syncope with bifascicular block may be caused by intermittent complete heart block, but competing ...
May 24, 2018 — New clinical study results demonstrate that an investigational algorithm, utilizing the accelerometer ...

 May 15, 2019
May 15, 2019
![A new infection risk scoring system has been developed based on data from the large PADIT Trial.[1] The new scoring system was presented as a follow up to that study during a late-breaking session at Heart Rhythm 2019, the Heart Rhythm Society's 40th Annual Scientific Sessions.](/sites/default/files/styles/content_feed_medium/public/PADIT_Infection_Risk_score.jpg?itok=O1-YAcMm)