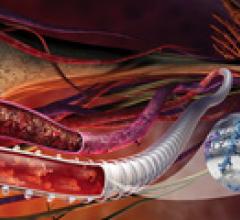
November 23, 2011 – The U.S. Food and Drug Administration (FDA) allowed marketing of the first system that can repair a ...
Stent Grafts
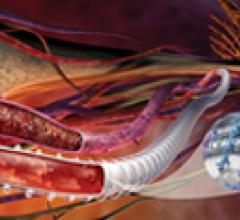
Stent grafts are used in transcatheter endovascular aortic repair (EVAR) procedures to seal aortic aneurysms. The grafts usually consist of a self-expanding stent frame that is covered with material to seal the vessel walls and prevent blood leaks feeding the aneurysm.
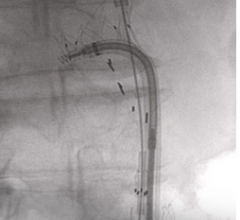
November 22, 2011 — The thoracic aorta can pose major problems for endovascular repair when there are tortuous segments ...
November 22, 2011 — The question remains whether patency rates for heparin-bonded polytetrafluoroethylene (PTFE) grafts ...
November 18, 2011 – W. L. Gore & Associates Inc. announced that it has received approval from the U.S. Food and Drug ...
November 4, 2011 — The U.S. Food and Drug Administration (FDA) has approved a stent graft system for patients with small ...
August 31, 2011 — Medtronic Inc. announced the start of its United States post-approval study of the Endurant AAA stent ...
August 19, 2011 - Endologix Inc. said a ruling this week will likely aid its defense in a stent graft patent litigation ...
August 12, 2011 — Endologix Inc. announced that the first American commercial implant of the company's AFX Endovascular ...
June 17, 2011 — Endologix Inc. received U.S. Food and Drug Administration (FDA) approval for AFX Endovascular AAA System ...
May 24, 2011 — Aptus Endosystems Inc., a medical device company developing advanced technology for endovascular aneurysm ...
Aortic dissection is rare, but when it occurs, a patient’s prognosis can be poor, even with timely medical diagnosis ...
March 14, 2011 – A vascular graft for hemodialysis access has received the CE mark. The Gore Propaten vascular ...
February 21, 2011 – The U.S. Food and Drug Administration has approved an endoprosthesis device for use on a lower ...
January 24, 2011 – A new device for treating peripheral artery disease (PAD) is now available in Europe. W.L. Gore ...
December 29, 2010 – The U.S. Food and Drug Administration (FDA) has approved a stent graft system for treating ...

 November 23, 2011
November 23, 2011