Bioresorbable stent (BRS) technology is not dead, but the unbridled enthusiasm seen two years ago for the technology has ...
Stents Drug Eluting
This channel includes news and new technology innovations for drug eluting stents (DES). These drug coated stents were developed to solve a frequent problem with bare metal stents, which can cause neointimal hyperplasia (scar tissue growth) in some patients. The antiproliferative drugs used on DES prevent the growth of tissue. One downside of DES is the requirement for patients to take long-term antiplatelet therapy to prevent the possible formation of clots on these stents. Newer generation DES use technologies help the vessels heal faster, which may allow reduce the duration of dual antiplatelet therapy (DAPT), or use a single drug, usually eliminating aspirin. This section includes news for both metallic and bioresorbable drug-eluting stents and related clinical trial data.
Professor Ian Meredith, MBBS, Ph.D., global chief medical officer and executive vice president, Boston Scientific ...
A discussion with Professor Ian Meredith, MBBS, Ph.D., global chief medical officer and executive vice president, Boston ...
October 4, 2018 – Investigators unveiled clinical data from the independent BIONYX and SORT OUT IX all-comers trials ...
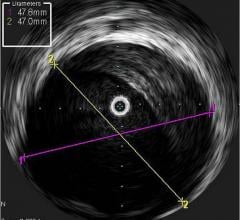
October 2, 2018 — Intravascular ultrasound (IVUS) guidance improved clinical outcomes over angiography guidance during d ...
October 1, 2018 — Recent results from the BIONYX randomized clinical study showed the novel, thin-strutted, polymer ...
October 1, 2018 — Boston Scientific announced that the U.S. Food and Drug Administration (FDA) has approved its ...
September 26, 2018 — Positive 12-month data from the late-breaking IMPERIAL trial was presented at the 2018 ...
May 31, 2018 – Late-breaking trial results presented at the EuroPCR Congress, May 21-24 in Paris, France, found the ...
May 31, 2018 — Two-year outcome data from the BIO-RESORT randomized controlled trial were presented in a late-breaking ...
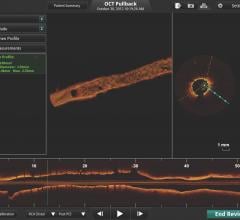
May 29, 2018 – Investigators recently unveiled clinical data from the independently run Onyx 1-Month OCT Study showing ...
May 29, 2018 – Medtronic plc announced the initiation of a U.S. clinical study to assess the safety and efficacy of drug ...
May 25, 2018 — Abbott announced it received approval from the U.S. Food and Drug Administration (FDA) for Xience Sierra ...
DAIC Editor Dave Fornell takes a tour of some of the most interesting new technologies on the expo floor at the 2018 ...
February 26, 2018 – Designed specifically for small vessels, Medtronic plc announced U.S. Food and Drug Administration ...

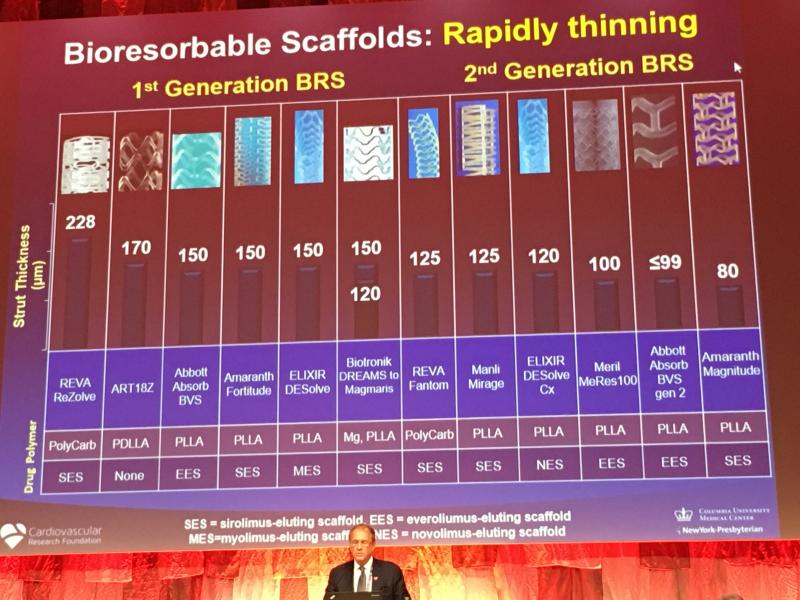
 October 12, 2018
October 12, 2018