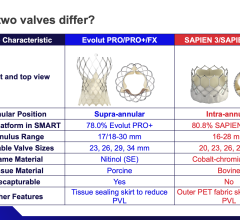
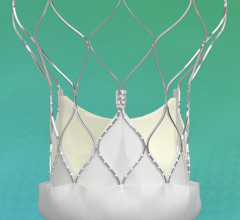
April 23, 2024 — Medtronic plc, a global leader in healthcare technology, today announced the launch of its latest innovation in cardiac surgery, the Avalus Ultra valve. This next-generation surgical ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
April 23, 2024 — Medtronic plc, a global leader in healthcare technology, today announced the launch of its latest ...
April 17, 2024 —CPR Therapeutics, Inc. (CPR-T), an early-stage medtech startup funded by the N.I.H and N.S.F to develop ...
April 16, 2024 — Vivasure Medical, a company pioneering novel fully absorbable technology for percutaneous vessel ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...
April 9, 2024 — UC Davis Health cardiology team members are among the first in the country to treat patients with tricus ...
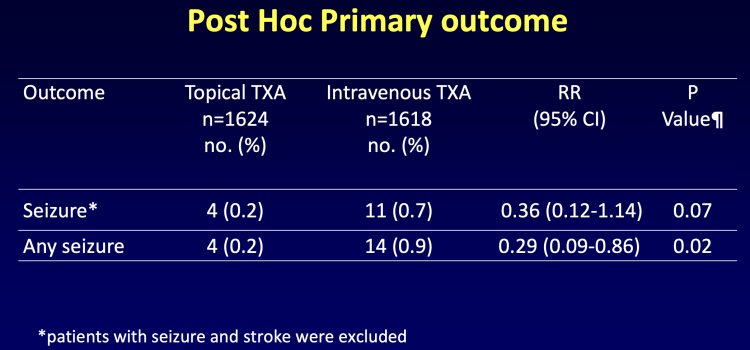
April 9, 2024 — Administering tranexamic acid (TxA), a drug used to reduce bleeding during heart surgery, topically ...
April 9, 2024 — People with a small aortic annulus, a part of the heart’s anatomy where the left ventricle meets the ...
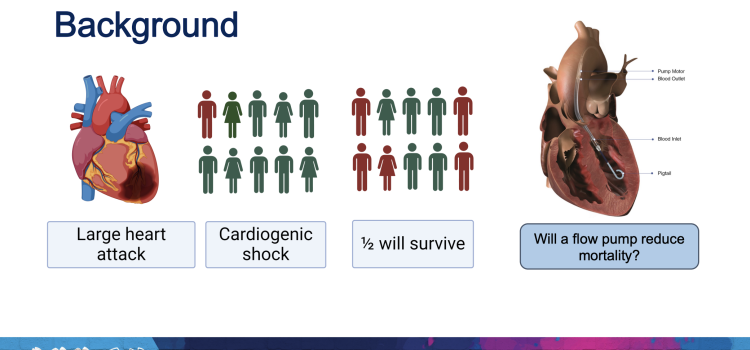
April 8, 2024 — Implantation of the Impella CP micro-axial flow pump in the hours after a heart attack significantly ...
April 4, 2024 — Osso VR, a leader in immersive procedural training, announces the release of a co-developed curriculum ...

Here is a Top 10 look at the content that was trending during the month of March on dicardiology.com:
1. CLS Health ...
April 2, 2024 — Abbott announced that the U.S. Food and Drug Administration (FDA) approved the company's first-of-its ...
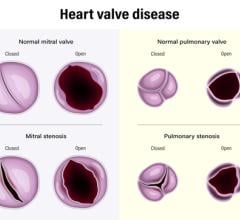
April 1, 2024 — Roughly 25,000 Americans die each year from valvular heart disease, but researchers from Rutgers Health ...
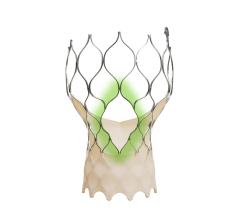
March 28, 2024 — Medtronic plc, a global leader in healthcare technology, announced that the United States Food and Drug ...
March 25, 2024 — In a groundbreaking medical advancement, three esteemed cardiologists from CLS Health, Dr. Bahaeddin ...
March 21, 2024 — The U.S. Food and Drug Administration (FDA) announced that Abiomed is recalling the Instructions for ...


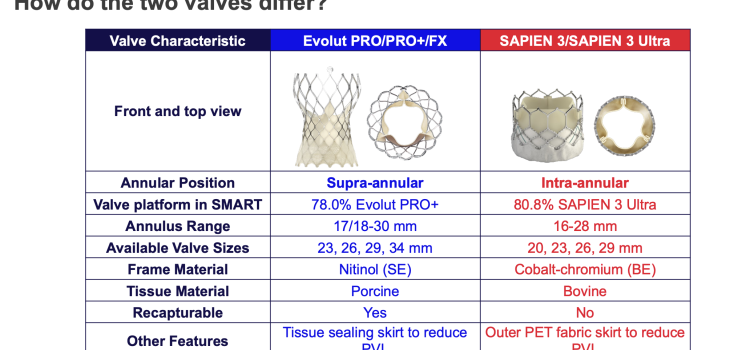

 April 23, 2024
April 23, 2024