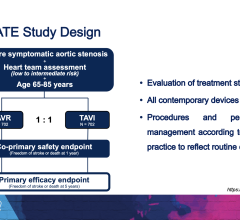
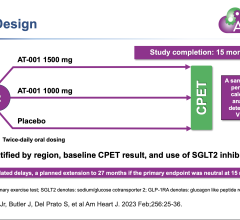
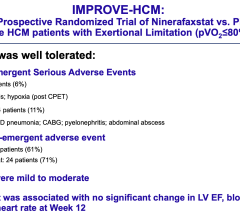
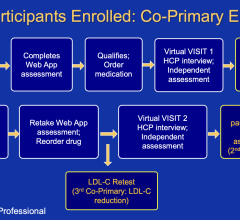
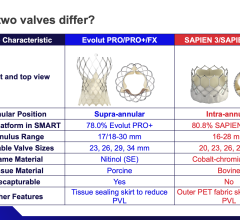
October 7,2014 — Amaranth Medical announced patient enrollment in multiple centers in Colombia, South America in MEND-II, a clinical trial to assess safety and feasibility of the company's Fortitude Sirolimus-Eluting Bioresorbable Scaffold in patients with symptomatic coronary artery disease. As seen with the first generation scaffold, preliminary patient results using optical coherence tomography (OCT) following implantation show that the scaffolds were fully apposed to the vessel wall achieving optimal acute lumen gain. Additional details of Amaranth's bioresorbable scaffold program will be presented at the 2014 Transcatheter Cardiovascular Therapeutics (TCT) Meeting during the didactic symposium.
"The Fortitude Sirolimus-Eluting Bioresorbable Scaffold is designed to have comparable mechanical strength and durability to our bare bioresorbable scaffold, while also providing the controlled release of sirolimus to reduce the risk of restenosis," said Kamal Ramzipoor, chief executive officer of Amaranth. "We look forward to advancing Fortitude through this clinical trial and initiating a study next year in Europe, both of which will support our planned application for CE Mark."
Complete 12-month clinical follow-up results on the 13 patients enrolled in MEND-I, Amaranth's clinical study of the bare Fortitude, will also be presented in the aforementioned didactic symposium at TCT. All patients enrolled in MEND-I have completed their one-year follow-up visits. This study met its primary endpoint of six-month incidence of target vessel failure with no incidents of stent thrombosis or mechanical failure. Importantly, late lumen loss, as confirmed by an independent core lab, was shown to be comparable to that of bare metal stents.
Juan F. Granada, M.D., executive director and chief innovation officer of the CRF-Skirball Center for Innovation (SCI) and primary investigator of the Amaranth clinical program said " while the Fortitude Sirolimus-Eluting Bioresorbable Scaffold is still in early stages of enrollment, it appears to have similar mechanical behavior but enhanced deliverability compared to the first-generation bare scaffold."
For more information visit: www.amaranthmedical.com

 April 16, 2024
April 16, 2024