September 24, 2010 - Trial data have established the safety of a reversible PAR-1 thrombin receptor antagonist in patients with acute coronary syndrome. Results from the LANCELOT ACS trial were presented at the Transcatheter Cardiovascular Therapeutics (TCT) 2010 meeting in Washington, D.C.
The study looked at atopaxar, by Eisai Pharmaceuticals.
“Overall we found that atopaxar was well tolerated and did not increase the risk of bleeding,” said lead researcher Michelle O’Donoghue, M.D., MPH, investigator, TIMI Study Group, Brigham and Women’s Hospital. “Future studies will be required to fully establish safety and efficacy of E5555, but PAR-1 blockade appears promising.”
In the trial, 603 subjects were randomized to one of the four treatment arms and 593 subjects took at least one dose of the study drug. The primary objective was to establish the safety and tolerability of E5555 in patients with acute coronary syndrome.
The rate of bleeding according to the CURE bleeding classification was 3.1 percent compared to 2.2 percent with the placebo.
The rate of cardiovascular death, heart attack or stroke was 3.3 percent (average of all active doses) compared to 5.6 percent with a placebo. The incidence of ischemia at 48 hours following a 400 mg loading dose was significantly lower at 18.7 percent compared to 28.1 percent with the placebo. Overall the drug was well tolerated, but dose-dependent transaminitis and relative QTc prolongation were observed with higher doses of the drug.
For more information: www.crf.org or www.eisai.com

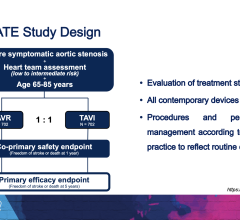
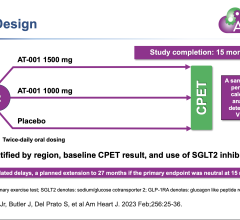
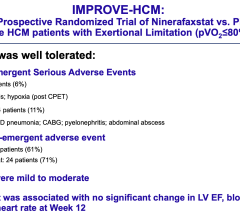
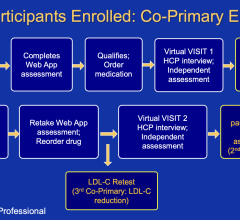
 April 16, 2024
April 16, 2024