A new way to examine stress and inflammation in the heart will help Parkinson’s researchers test new therapies and explore an unappreciated way the disease puts people at risk of falls and hospitalization.
Medtronic plc recently received U.S. Food and Drug Administration (FDA) approval for a less-invasive implant approach of its HVAD System, a left ventricular assist device (LVAD) for patients with advanced heart failure. The HVAD System is the smallest commercially available LVAD according to Medtronic, and the only LVAD approved in the U.S. for implant via thoracotomy, a small lateral, surgical incision between the patient's ribs on the left side of the chest.
Endologix Inc. announced one-year results from the LUCY (Evaluation of FemaLes who are Underrepresented Candidates for Abdominal Aortic AneurYsm Repair) registry at the 2018 Society for Vascular Surgery (SVS) Annual Meeting, June 21-23 in Boston. The LUCY study is the first to prospectively evaluate endovascular aneurysm repair (EVAR) outcomes in women who have more complex aortic anatomy and, subsequently, have worse reported outcomes than men undergoing EVAR. The results of the LUCY one-year data expand on the 30-day results presented last year, showing that at least 28 percent more women are eligible for minimally-invasive EVAR when using the Ovation Abdominal Stent Graft System than when using other EVAR systems.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
New research published in Experimental Physiology has indicated potential differences in heart health benefits of exercise intensity in teenagers. Teenage years are an important stage of life, with research suggesting it is a time during which heart diseases start to develop. These findings indicate that teenagers who participate in high intensity exercise have lower blood pressure. This may lead to a lower risk of developing heart disease later in life, but this requires confirmation with further research.
Boston Scientific Corp. recently announced a definitive agreement to acquire Cryterion Medical Inc., a privately-held company developing a single-shot cryoablation platform for the treatment of atrial fibrillation (AF). The addition of this cryoballoon platform positions the company as the first to have both cryothermal and radiofrequency (RF) single-shot, balloon-based ablation therapies in its portfolio.
July 13, 2018 – iSchemaView has signed Diagnostic Imaging Australia (DIA) to be the exclusive distributor for the RAPID ...
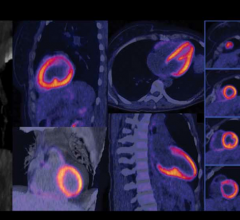
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Intact Vascular Inc. announced the publication of the iDissection Classification trial results in the current issue of Journal of Invasive Cardiology.
Zebra Medical Vision has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Coronary Calcium Scoring algorithm. The algorithm, capable of automatically calculating a patient’s Agatston equivalent coronary calcium score from an electrocardiogram (ECG)-gated computed tomography (CT) scan, provides physicians with important data used in the assessment of the risk for coronary artery disease.
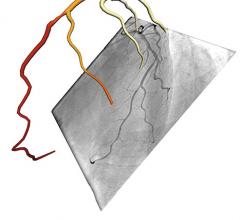
CathWorks announced the approval of a new Current Procedural Terminology (CPT) code 0523T for non-invasive, 3-D FFRangio-enabled interpretation of possible atherosclerotic stenosis during coronary angiography interventions. The company said the code issuance is a major step forward in helping physicians objectively and cost-effectively determine if percutaneous coronary intervention (PCI) is indicated and revascularization has occurred in every coronary angiography procedure.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...

Placement of implantable cardioverter defibrillators (ICDs) not meeting Centers for Medicare and Medicaid Services (CMS) National Coverage Determination criteria declined following the announcement of a U.S. Department of Justice (DOJ) investigation into potential overuse of such devices. This conclusion was drawn from a recent study of hospitals participating in the NCDR ICD Registry.
French-based CorWave announced that its CALYPSO program has received 14 million euros to develop CorWave Neptune, a new type of cardiac support to improve the management of patients with severe heart failure. The CALYPSO Research and Development (R&D) program, with a total budget of 25 million euros over 4 years, will be partially financed with 14 million euros support from the Programme d'Investissements d'Avenir (Future Investments Program), managed by the Secrétariat Général pour l’Investissement (General Secretariat for Investment – SGPI) and operated by Bpifrance.
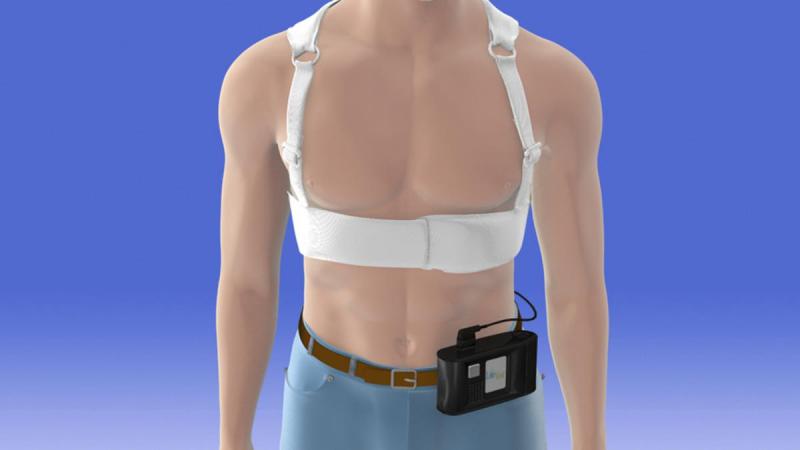
Following the Vest Prevention of Early Sudden Death Trial (VEST) presentation at the 2018 American College of Cardiology ...
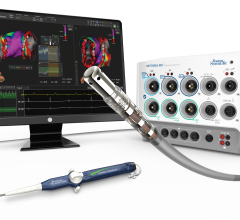
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
July 10, 2018 — Evanston, Ill.-based startup Visura Technologies has received 510(k) clearance from the U.S. Food and ...
Ori Ben-Yehuda, M.D., executive director of the Cardiovascular Research Foundation Clinical Trial Center, discusses the ...
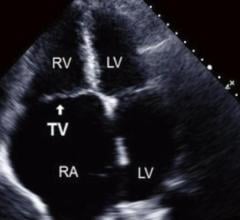
I attended the American Society of Echocardiography (ASE) 2018 meeting in June and have the following takeaways on ...

 July 17, 2018
July 17, 2018