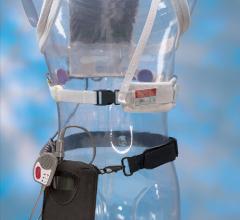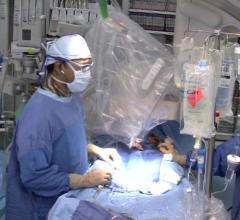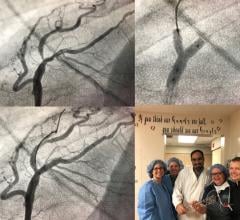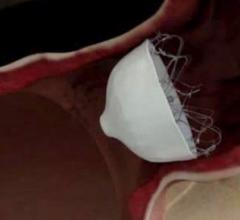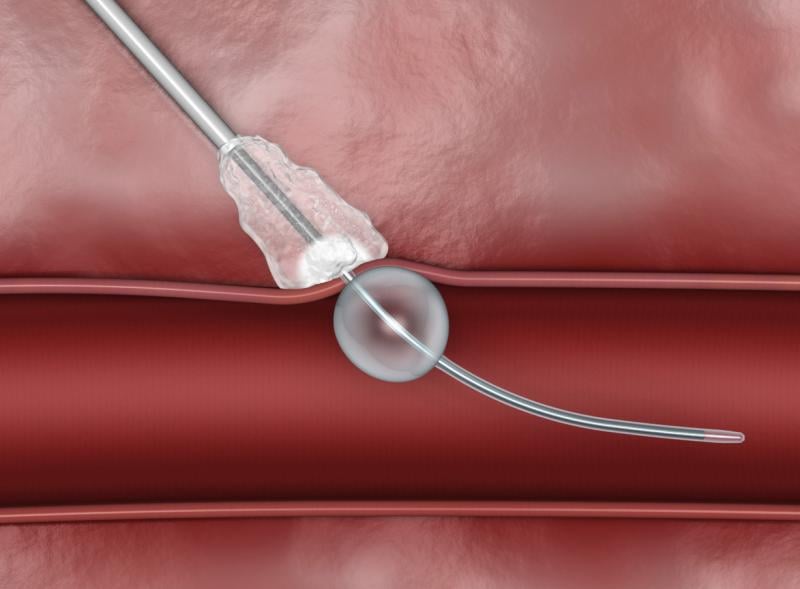
While manual compression remains the gold standard for hemostasis of catheterization vascular access site arteriotomies ...
The U.S. Food and Drug Administration (FDA) announced last week it is providing information and recommendations regarding the Zoll LifeVest 4000 due to concerns that the device may fail to deliver treatment to the patient if the device is not replaced soon after displaying "Call for service: Device has a problem that may require service. Call Zoll for service, Message Code 102." Failure to contact Zoll and immediately replace the device after Message Code 102 appears on the device screen may result in serious patient harm or death of the patient because the device may fail to deliver therapy appropriately when needed.
January 22, 2018 — Baxter International Inc. announced the U.S. Food and Drug Administration (FDA) approval of ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Ligand Pharmaceuticals Inc. announced initiation of a program to develop contrast agents with reduced renal toxicity. Through this new, internally-funded program, Ligand intends to advance products toward proof-of-concept, followed by selling or out-licensing them for further development and commercialization. The program will leverage Ligand’s patented Captisol technology, as well as data and intellectual property obtained through its acquisition of Verrow Pharmaceuticals, a privately-held Lenexa, Kansas-based medical invention company that Ligand acquired in January 2018 for $2 million in cash plus earnouts.
Abbott last week announced CE Mark approval for the company's new Advisor HD Grid Mapping Catheter, Sensor Enabled, a product designed to advance cardiac mapping during cardiac ablation to treat patients with complex cardiac arrhythmias. With the European launch of this latest addition to Abbott’s electrophysiology portfolio, the company is offering physicians a mapping catheter with what it calls a first-of-its-kind grid configuration of electrodes for improved data collection that supports the creation of high-density mapping of cardiac tissue to support optimal treatment for patients.
The U.S. Food and Drug Administration (FDA) announced that International Laboratories LLC is voluntarily recalling Lot# 117099A of Clopidogrel Tablets, USP 75 mg, packaged in bottles of 30 tablets, to the consumer level due to mislabeling. The product is labeled as Clopidogrel Tablets USP, 75 mg but may contain Clopidogrel 75mg or Simvastatin Tablets USP 10 mg.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
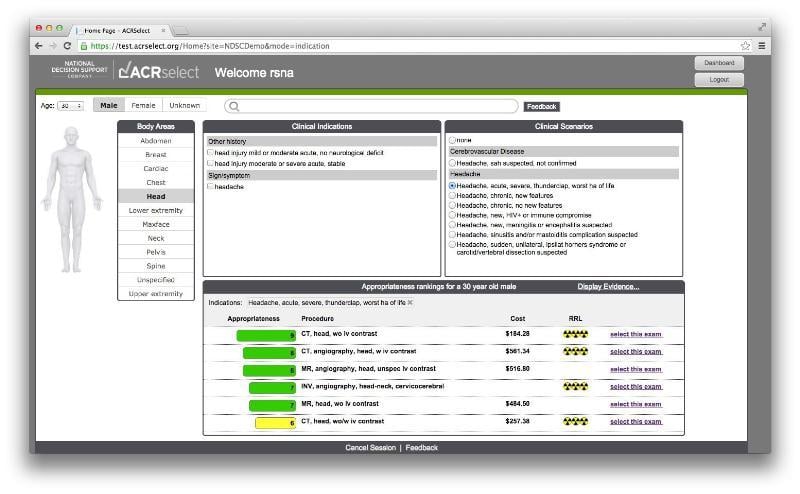
January 18, 2018 — Change Healthcare announced the acquisition of National Decision Support Company (NDSC), a leader in ...
January 18, 2018 – West Hills Hospital & Medical Center, a full-service acute care facility, announces the first ...
January 18, 2018 – Johnson & Johnson Medical Devices Companies announced that Biosense Webster, Inc., a worldwide leader ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Here is a list of what I think were some of the top interventional technologies discussed at the 2017 Transcatheter ...
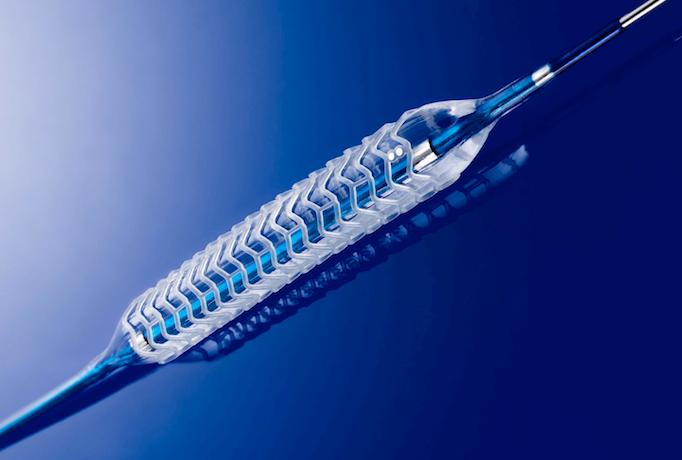
There was no shortage of interest in bioresorbable stent technologies at the many sessions offered on this topic or ...
Philips recently announced the launch of Technology Maximizer, a cross-modality program designed to boost the clinical capabilities and performance of imaging equipment through proactive upgrades. Technology Maximizer provides confidence to radiology and cardiology department leaders and clinicians that their systems are up to date and compliant at a fraction of the cost of individual upgrades, according to Philips.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
The Society for Vascular Ultrasound (SVU), the Society for Vascular Surgery (SVS) and Medstreaming-M2S announced the development of the Vascular Quality Initiative (VQI) Vascular Ultrasound Registry. This Registry represents an expansion of the SVS VQI, which will combine noninvasive (vascular ultrasound) testing data with vascular treatment and outcomes data, making it possible to analyze the relationships between diagnosis and care provided to patients with vascular disease.
Secant Group, in partnership with its sister company SanaVita Medical, announced new technology to advance cardiovascular regenerative medicine with the development of a synthetic, small-bore vessel that encourages endogenous regeneration and new vessel formation. The technology is based on the company’s textile forming capabilities that can produce a hollow lumen construct infused with Secant’s proprietary Regenerez bioresorbable polymer technology. The new small-bore vessel supports the regeneration of new vascular tissue structures without the need for cell seeding or biologic growth promoters.
January 16, 2018 — Researchers from the NUST MISIS Engineering Center for Industrial Technologies in Russia have ...

 January 22, 2018
January 22, 2018