The vacancy rate for radiographers increased to 4.2 percent in 2017, according to the latest American Society of Radiologic Technologists (ASRT) Radiologic Sciences Staffing and Workplace Survey.
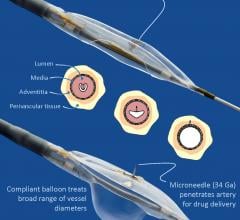
Mercator MedSystems Inc. announced the first patient enrollment into the TANGO (Temsirolimus Adventitial Delivery to Improve Angiographic Outcomes Below the Knee) clinical trial. TANGO will study the effects of using Mercator’s proprietary Bullfrog Micro-Infusion Device for the adventitial delivery of the drug Torisel (temsirolimus) after revascularization of lesions below the knee (BTK) in patients with critical limb ischemia (CLI).
A new Varicose Vein Registry has begun producing useful outcomes information, as reported in the May edition of the Journal of Vascular Surgery: Venous and Lymphatic Disorders. The registry is a joint effort by the Society for Vascular Surgery, the Vascular Quality Initiative and the American Venous Forum. It was launched in January 2015 and was set up to track systematically the outcomes of various treatments for varicose veins, ultimately providing guidance for both consumers and treating physicians.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Boston Scientific announced results from the RANGER SFA trial for the Ranger Paclitaxel-Coated PTA Balloon Catheter at the Charing Cross Symposium, in London. Data demonstrated that the drug-coated balloon (DCB) exhibited both a high rate of primary patency and freedom from target lesion revascularization (TLR) at 12 months, reducing the need for re-interventions to re-establish flow in previously blocked blood vessels.
The issues surrounding congenital coronary anomalies and their effect on sudden death are complex. Researchers are still trying to fully understand anomalous aortic origin of a coronary artery (AAOCA) and its relationship to adverse health outcomes in humans, especially children. Using the most up-to-date literature, as well as the input of leading experts in the field, the American Association for Thoracic Surgery (AATS) has released practical guidelines for the identification and treatment of AAOCA, including an overview of the latest data surrounding population-based risk.
Repeated cycles of weight loss and gain may be linked to higher risk for stroke, heart attack and death in people with pre-existing coronary artery disease, according to a study published online in the New England Journal of Medicine.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
April 25, 2017 — Corindus Vascular Robotics Inc. announced a strategic partnership with BLOXR Solutions, provider of ...
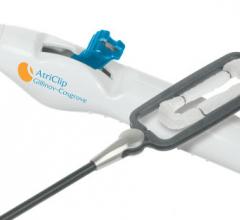
AtriCure Inc. announced it has sold more than 100,000 AtriClip Left Atrial Appendage Exclusion System devices worldwide. This makes it the most widely used of all devices for excluding the left atrial appendage (LAA), according to the company.
April 24, 2017 — Offering a full range of advanced clinical applications for researchers, Toshiba Medical will ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
A recent study conducted at Centre Hospitalier Universitaire (CHU) of Saint-Étienne, France validated the advantages of the Niobe ES magnetic navigation system over the Niobe II system in terms of procedure and fluoroscopy times for atrial fibrillation (AF) ablation procedures. The study was published in the International Journal of Cardiology and represents the first comparison study of Stereotaxis’ latest-generation remote magnetic navigation system to its predecessor.
Physio-Control announced April 19 that the company’s HeartSine samaritan PAD 360P (SAM 360P) fully automatic external defibrillator (AED) is now available for sale in the United States, having received U.S. Food and Drug Administration (FDA) premarket approval (PMA).
The Structural Heart Program at Princeton Baptist Medical Center, (Birmingham, Ala.) recently became the first center in the Southeast to implant the newly U.S. Food and Drug Administration (FDA)-approved CoreValve Evolut Pro.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
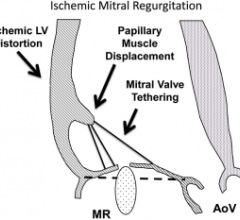
April 20, 2017 — Mitral regurgitation can occur in up to 50 percent of patients with ischemic heart disease and even ...
Cardiovascular Systems Inc. (CSI) announced April 18 it had initiated a voluntary recall of its 7-10014 Saline Infusion Pump. CSI initiated a customer communication of the recall by letter and informed customers that they may continue to use the affected Saline Infusion Pumps until they receive a replacement.
Murj Inc., a digital health company that helps manage implantable cardiac device data, announced more than $4.5 million in financing. True Ventures led the most recent Series A financing. Social Capital joined the Series A, along with investors from Murj’s seed round.


 April 27, 2017
April 27, 2017