March 21, 2017 — In the late-breaking ABSORB III Trial two year results presented at the American College of Cardiology ...
March 20, 2017 — Teleflex Inc. recently announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and U ...
March 20, 2017 — Agfa HealthCare announced the release a new version of its Enterprise Imaging for Cardiology platform ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
March 20, 2017 — Cardiologists can now access the advanced ultrasound imaging technology needed for fast and confident ...

March 20, 2017 — For patients experiencing angina (chest pain) or a heart attack, instantaneous wave-free ratio (iFR) ...
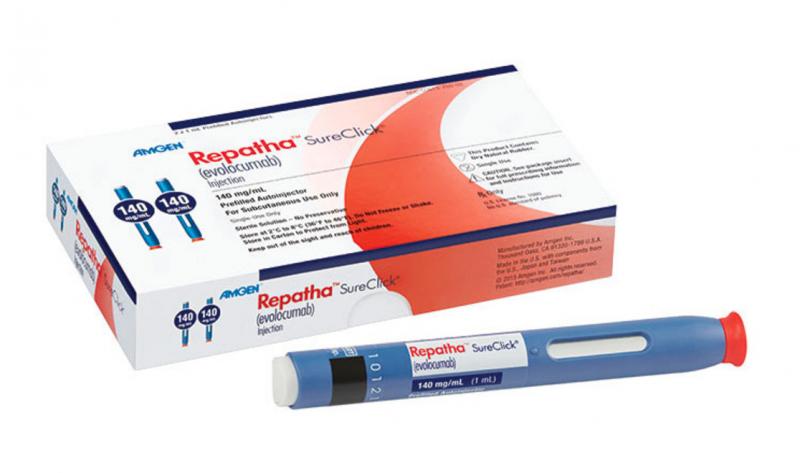
March 20, 2017 — The most important late-breaking pharmaceutical trial at the 2017 America College of Cardiology annual ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
March 17, 2017 - Transcatheter aortic valve replacement (TAVR) was found to be noninferior to surgical aortic valve ...
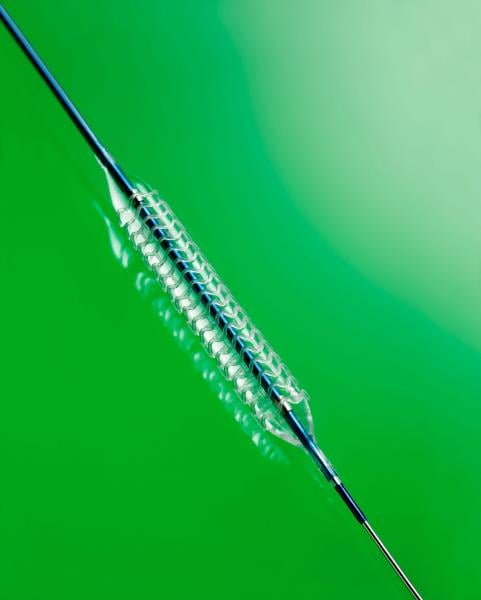
W. L. Gore & Associates Inc. recently announced the Health Canada approval of the Gore Tigris Vascular Stent, a dual-component stent with a fluoropolymer/nitinol design. The device, which gained CE Mark approval in 2011, is a third-generation, self-expanding stent that was designed explicitly to improve anatomical conformability with the natural movement of the knee when treating peripheral artery disease (PAD).
Alcohol abuse increases the risk of atrial fibrillation, heart attack and congestive heart failure as much as other well-established risk factors, according to a study published recently in the Journal of the American College of Cardiology.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
During the 66th Annual Scientific Session & Expo of the American College of Cardiology (ACC), March 17-19 in Washington, D.C., Siemens Healthineers will showcase technologies that integrate images and information at every step of the treatment process, with a theme of “Making Complex Simple.”
A new document from the American Society of Echocardiography (ASE) provides a comprehensive update to guide clinicians in best practices for assessing all forms of valvular regurgitation.
Medtronic plc announced U.S. Food and Drug Administration (FDA) 510(k) clearance for its Reveal LINQ Insertable Cardiac Monitor (ICM) with TruRhythm Detection. The advanced cardiac monitor offers improved accuracy to better identify abnormal heartbeats.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
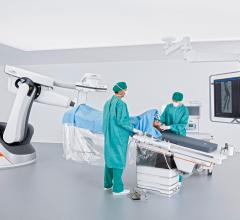
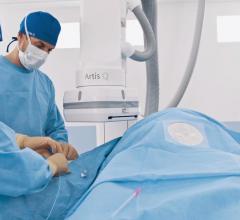
The U.S. Food and Drug Administration (FDA) has cleared the Artis pheno robotic C-arm angiography system from Siemens Healthineers created for use in minimally invasive interventional procedures.
Using marijuana raises the risk of stroke and heart failure even after accounting for demographic factors, other health conditions and lifestyle risk factors such as smoking and alcohol use, according to new research. The study is scheduled for presentation at the American College of Cardiology’s 66th Annual Scientific Session, March 17-19 in Washington, D.C.
Pam Rush, RN, MS, clinical program director, cardiovascular service line, and Craig Strauss, M.D., MPH, medical director ...

 March 21, 2017
March 21, 2017