January 12, 2017 — Bayer Healthcare has initiated a recall of all its Medrad Intego PET Infusion System Source ...
According to a recent study by the Harvey L. Neiman Health Policy Institute, the last two decades have seen a substantial decline in new enteral access procedures in the Medicare population. The study, published online in November in the Journal of Vascular and Interventional Radiology (JVIR), also found that maintenance services have increased, with radiologists and emergency physicians surpassing gastroenterologists and surgeons as the leading providers of those procedures.
January 11, 2017 — Lombard Medical Inc. recently announced approval from the Japanese Ministry of Health, Labour and ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
For patients with atrial fibrillation, the most common form of heart arrhythmia, a main goal of treatment is stroke prevention. As a result, most Afib patients are prescribed a blood thinner such as warfarin, also known by the brand name Coumadin, to combat the potential for blood clots that could lead to stroke. But warfarin is tough to manage, and some patients have trouble adhering to any medication. A new research letter published in JAMA Cardiology finds Afib patients are even more likely to discontinue warfarin therapy if they’ve had a recent procedure done to address their arrhythmia.
January 11, 2017 — Avinger Inc. recently announced the U.S. launch of an enhanced version of the company’s Lightbox ...
BioVentrix Inc. announced in December the first closed-chest Revivent TC TransCatheter Ventricular Enhancement System procedure in Germany since receiving CE mark certification. The Less Invasive Ventricular Enhancement (LIVE) procedure was performed by interventional cardiologists Christian Frerker, M.D. and Tobias Schmidt, M.D., and by cardiothoracic surgeon Ralf Bader, M.D., at Asklepios Klinik St. Georg in Hamburg, Germany. The St. Georg Heart team is led by Prof. Dr. Karl-Heinz Kuck, who is also the chairman of the Department of Cardiology and Electrophysiology.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Medinol announced in December positive twelve-month clinical results from the BIONICS study. The study was conducted to evaluate EluNIR, Medinol's novel coronary stent system and the first ever elastomer-coated drug eluting stent (eDES), according to the company.
Ischemic heart failure from previous heart attacks and coronary artery disease is the leading cause of death in the world, affecting more than 12 percent of the world’s population, according to the World Health Organization. Stem cell therapy has been conducted to try to repair heart damage from ischemic heart failure, but in previous studies, the two types of stem cells (autologous bone marrow derived mesenchymal cells [MSCs] and endomyocardial biopsy derived c-kit+ Cardiac Stem Cells [CSCs]) have been used in separate trials. In a first-in-the-world study, the Minneapolis Heart Institute Foundation (MHIF) is about to begin the CONCERT study, led by Principal Investigator Jay Traverse, M.D. The study will use MSCs and CSCs together to learn if the combination would be more successful than using either alone based on pre-clinical studies in swine demonstrating an enhanced synergistic effect of the combination.
January 10, 2017 — Teleflex Inc. announced that its Arrow VPS Rhythm Device with optional TipTracker technology has been ...
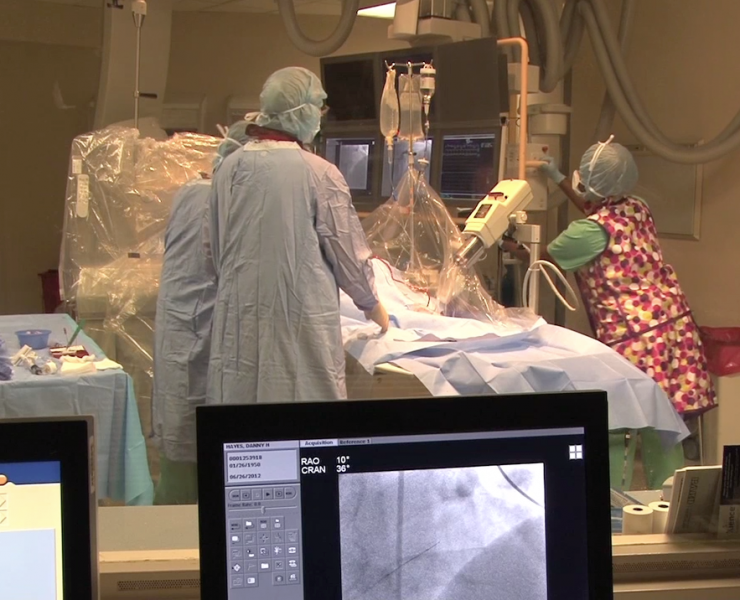
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
Effective July 1, 2017 for Medicare heart attack patients, the the Centers for Medicare and Medicaid Services (CMS) will ...
BioCardia Inc. announced in December the issuance of United States Patent No. 9,517,199 relating to a method of delivering cells to patients who have chronic myocardial infarcts. This new patent follows United States Patent No. 9,504,642, issued to BioCardia two weeks prior.
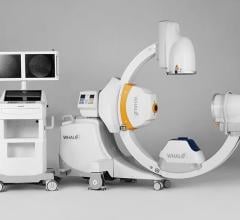
Whale Imaging Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance of the G-Arm Duo. The Duo is the next generation of the G-Arm, with major improvements including axial tilt and greater table access.
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
Lumedx Corp. announced at the end of November that two Marshall Medical Centers hospitals have gone live with the first phase of a cardiovascular information system (CVIS) deployment. The two hospitals now using Lumedx CVIS software are Marshall Medical North in Guntersville, Ala.; and Marshall Medical South in Boaz, Ala.
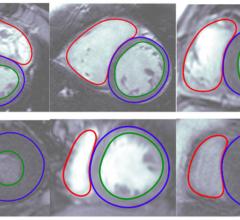
Arterys has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Arterys Cardio DL application. Arterys Cardio DL is the first technology to be cleared by the FDA that leverages cloud computing and deep learning in a clinical setting.

January 9, 2017 — The U.S. Food and Drug Administration (FDA) issued a safety communication today concerning patient ...


 January 12, 2017
January 12, 2017