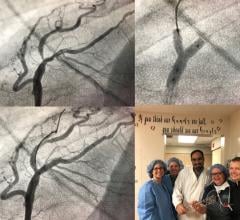
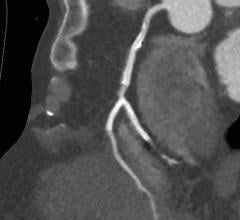
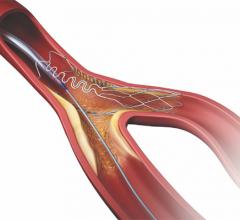
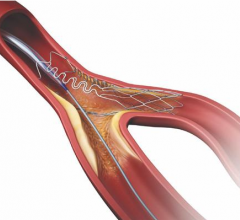
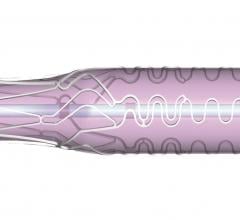
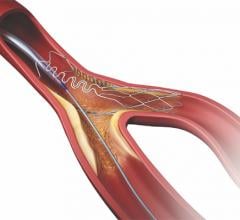
September 21, 2022 — Medtronic announced today that it has received Food and Drug Administration (FDA) approval for the treatment of non-left main bifurcation lesions utilizing the provisional ...
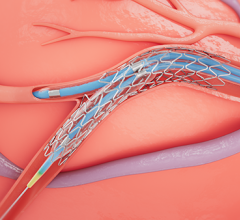
Stents Bifurcation
Patients with coronary artery disease where a lesion is located at a vessel bifurcation and extends into the side branch can be difficult to treat in a cath lab. The conventional treatment for bifurcations is to either used a single stent and leaving part of the vessel untreated, or use of two stents. Use of two stents can "cage" or "jail" the side branch vessel because of the stent struts in the main vessel. This might prevent future access to treat the side branch vessel. This channel offers news on bifurcation treatment strategies and development of dedicated side branch stents.
This is an animation showing how the dedicated bifurcation stent developed by Advanced Bifurcation Systems (ABS) is ...
May 29, 2018 – Medtronic plc announced the initiation of a U.S. clinical study to assess the safety and efficacy of drug ...
January 18, 2018 – West Hills Hospital & Medical Center, a full-service acute care facility, announces the first ...
Philippe Genereux, M.D., co-director of the structural heart program at the Gagnon Cardiovascular Institute at ...
November 10, 2017 — Cordis, a Cardinal Health company, recently unveiled a comprehensive interventional cardiology portf ...
November 1, 2017 — The double kissing (DK) crush two-stent technique was associated with a lower rate of target lesion ...
September 21, 2017 — Cardinal Health and Tryton Medical Inc. announced that the Tryton coronary Side Branch Stent was ...
March 31, 2017 — Tryton Medical Inc. recently announced that the first U.S. commercial case using the Tryton Side Branch ...
This video, provided by Tryton, demonstrates the implantation of the Tryton Side Branch Stent. It became the first ...
March 6, 2017 – The U.S. Food and Drug Administration (FDA) has granted pre-market approval (PMA) for the Tryton Side ...
October 28, 2016 – Tryton Medical Inc., a primary developer of stents to treat coronary bifurcation lesions, and
...
October 14, 2015 — Tryton Medical Inc. announced results from the pivotal Tryton Confirmatory Study confirming the ...
October 2, 2015 — Tryton Medical Inc. announced that results of a post hoc analysis of the pivotal Tryton Randomized ...

March 3, 2015 — Tryton Medical Inc. announced that results from the company’s pivotal clinical trial for the Tryton Side ...

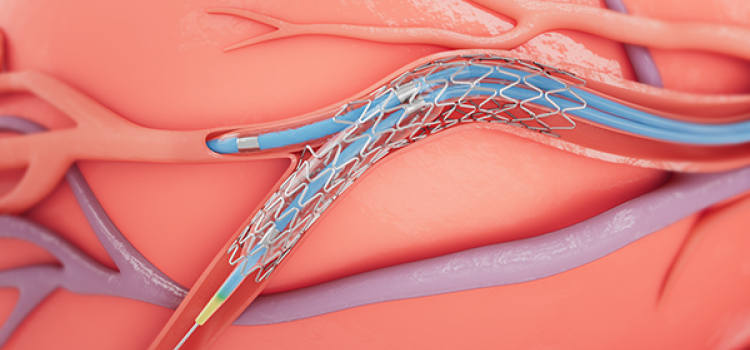


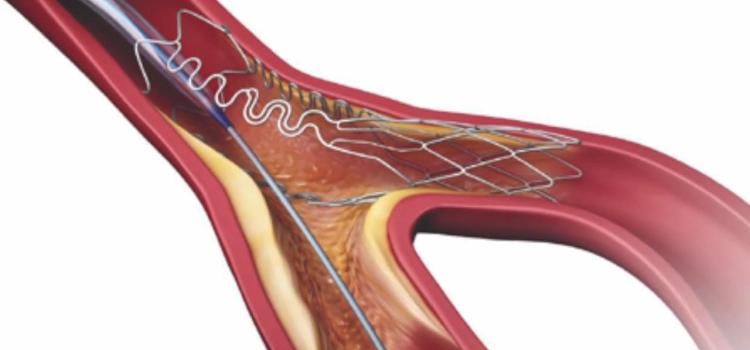
 September 21, 2022
September 21, 2022