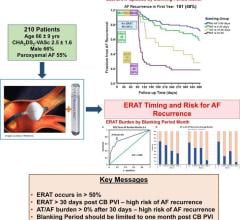
April 18, 2024 — New evidence-based research calls into question the conventional three-month blanking period immediately after atrial fibrillation (AF) ablation, when early occurrences of AF are ...
Atrial Fibrillation
This channel includes news and new technology innovations for the treatment of atrial fibrillation, also referred to as AF or afib. AF is a cardiac arrhythmia caused by irregular and often rapid heart rate. It is caused by the upper chambers (the atria) beating irregularly and uncoordinated with the lower ventricle chambers of of the heart. Symptoms include weakness with heart palpitations and shortness of breath. The conditional can lead to an increased risk of stroke and heart failure. AF episodes can cause the blood in the atria to stagnate and form clots, usually within the left atrial appendage (LAA). The clots can flow to the brain and cause a stroke. Treatments include anticoagulation therapy to dissolve clots, catheter or surgical ablation and LAA occlusion.
April 18, 2024 — New evidence-based research calls into question the conventional three-month blanking period ...
April 10, 2024 — An international consensus statement on how to treat atrial fibrillation with catheter or surgical ...
April 5, 2024 — Johnson & Johnson and Shockwave Medical, Inc. today announced that they have entered into a definitive ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
March 28, 2024 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & Johnson ...
March 26, 2024 — UC San Diego Health is the first health system in San Diego to successfully implant the world’s first ...
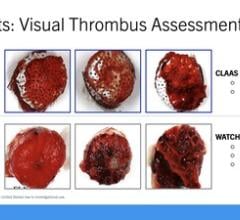
March 18, 2024 — Conformal Medical, Inc. announced that the CLAAS System was featured in a podium presentation at the Ca ...
For over a decade, the cardiac cryoablation industry has seen little in the way of technological advancements. Yet ...
February 27, 2024 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & ...
February 12, 2024 — BIOTRONIK, a leader in implantable medical device technology, announced today they will solely ...
In the United States, the options currently available for cardiac ablation use thermal mechanisms to ablate tissue and ...
February 7, 2024 — Combining brain stimulation with intense physical rehabilitation helped stroke survivors recover ...
February 6, 2024 — Cortex announced the initiation of its RESOLVE-AF trial (NCT05883631), a study formally launched in ...
February 2, 2024 — Biosense Webster, Inc., a global leader in cardiac arrhythmia treatment and part of Johnson & Johnson ...
When the patients of Michael Boler, M.D. need cardiac monitoring, the Holter monitor is no longer his first choice. “The ...
January 31, 2024 — Boston Scientific Corporation announced it has received U.S. Food and Drug Administration (FDA) appro ...
January 18, 2024 — Abbott announced the first global procedures have been conducted using the company's new Volt Pulsed ...
January 8, 2024 — University Hospitals (UH) Harrington Heart & Vascular Institute recently became the first center in ...

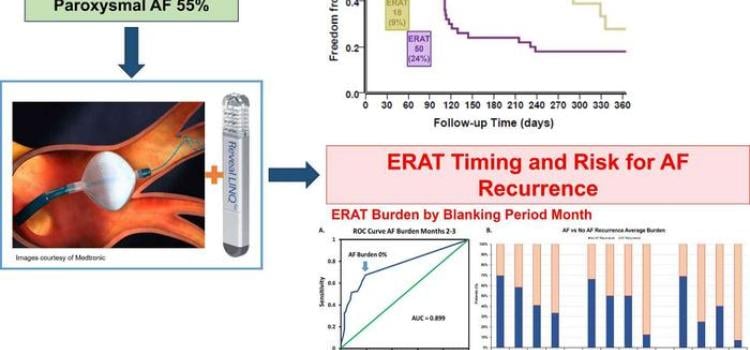



 April 18, 2024
April 18, 2024