GE Healthcare announced that it has filed a supplemental new drug application (sNDA) that will allow the company to manufacture Optison (Perflutren Protein-Type A Microspheres Injectable Suspension, USP), within its own facility. Optison is a contrast agent that may improve the visualization of the left ventricular border — an area of the heart that is critical to see in order to assess and diagnose certain heart diseases. Upon approval, GE Healthcare will provide supply of Optison to the U.S. market from its manufacturing facility in Oslo, becoming the only contrast media manufacturer to supply its own stock for the United States.
nContact Inc. announced that the U.S. Food and Drug Administration (FDA) has cleared the Company’s modifications to its VisiTrax cardiac ablation device, EPi-Sense, now with embedded sensing capability. This next generation of technology includes sensors along the ablation device, which provide real-time feedback to physicians conducting cardiac ablation procedures.
CardioMEMS Inc. has been honored as the 2012 recipient of the Intel Innovation Award. Founder and CEO Jay Yadav, M.D., was presented with the award on Dec. 4, 2012 during the annual Health IT Leadership Summit held at the historic Fox Theatre in Atlanta, Ga.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...

CardioKinetix Inc. announced the enrollment of the first patients in PARACHUTE IV, the randomized pivotal U.S. trial of the minimally invasive Parachute Ventricular Partitioning Device for the treatment of heart failure.
GSI Group Inc., a leading supplier of laser-based solutions, precision motion and optical technologies to global industrial, medical, electronics and scientific markets, announced that it has acquired NDS Surgical Imaging (NDS), a San Jose, Calif.-based global leader in surgical and radiology displays and related peripherals, for $82.5 million in cash, subject to customary closing adjustments.
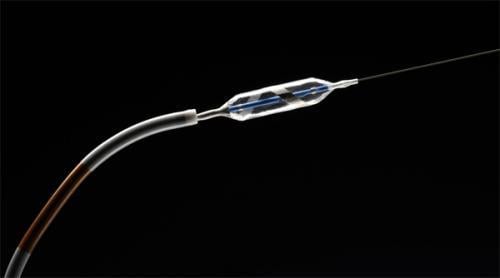
Covidien began the commercial launch of the OneShot Renal Denervation System, an over the wire balloon-based irrigated ablation catheter technology for the treatment of hypertension.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

The American Medical Association (AMA) submitted formal comments to the Office of the National Coordinator for Health Information Technology (ONC) on the Health IT Policy Committee’s proposal for Stage 3 of the Medicare/Medicaid meaningful use electronic health record (EHR) program requirements.
Open heart surgery may be a dying art, as new valve replacement techniques offer life-changing treatment without the need for invasive procedures, states a new report by healthcare experts GlobalData.
Lantheus Medical Imaging Inc. has added a low-enriched uranium (LEU) TechneLite (technetium Tc 99m Generator) generator to the its nuclear imaging product portfolio. Lantheus’ LEU TechneLite generator is the first technetium-99m (Tc-99m) generator in the United States that contains molybdenum-99 (Mo-99) produced from at least 95 percent LEU. With greater access to LEU Mo-99 through its supply chain diversification strategy, Lantheus can now move closer to its goal of eventually eliminating Highly Enriched Uranium (HEU)-sourced Mo-99 from its supply chain. Lantheus’ first LEU TechneLite generator was shipped on Jan. 7, 2013.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
January 16, 2013 — CardioLogical Solutions, a new cardiovascular device company, announced it has initiated operations. CardioLogical Solutions represents the merger of two independent companies, Emboline and VasoStitch.
January 16, 2013 — Rex Medical L.P. announced that it has received 510(k) Clearance from the U.S. Food and Drug Administration (FDA) for the Cleaner15 Rotational Thrombectomy System.
January 16, 2013 — Americans' cardiovascular health varies greatly from state to state, according to new research in the Journal of the American Heart Association (JAHA). The study is the first to assess cardiovascular health at the state level.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
The U.S. Food and Drug Administration (FDA) has cleared St. Jude Medical’s new version of its Merlin.net Patient Care Network (PCN). The secure, Internet-based remote care system is for patients with implanted implantable cardioverter defibrillators (ICDs), cardiac resynchronization therapy (CRT) devices and pacemakers.
January 16, 2013 — Medtronic’s CareLink Express service is a remote monitoring system that enables clinicians in healthcare facilities to quickly obtain data regarding the status of Medtronic implanted cardiac devices, facilitating faster treatment decisions.

January 16, 2013 — Just weeks after the Food and Drug Administration (FDA) approved Cook Medical’s Zilver PTX drug-eluting peripheral stent, Riverside Methodist Hospital in Columbus, Ohio, has treated the first patient with the device as part of Cook’s U.S. commercial launch.

 January 18, 2013
January 18, 2013