Building teams that include advanced practice providers can help cardiovascular practices meet the challenges of modern practice, according to a new health policy statement by the American College of Cardiology (ACC). These challenges include workforce shortages, an aging patient population with growing complexities in care and a payment system in transition.
NeoStem Inc. presented updated efficacy and safety results from the one-year follow-up for its Phase 2 PreSERVE study and additional analyses of certain functional tests at the American College of Cardiology’s 64th annual scientific session and expo in March. The one-year follow-up results are defined as all data accumulated until the last patient enrolled completed 12-month follow-up. Thus, the results actually represent data from patients with a median follow-up of 18 months.
Medtronic plc announced the first in-office implant of its miniaturized cardiac monitor as part of the Medtronic Reveal LINQ In-Office 2 (RIO 2) Study. One of the world's smallest cardiac monitors, the Reveal LINQ insertable cardiac monitor (ICM) was successfully implanted in an office setting at Scripps Clinic in La Jolla, California, by cardiologist John Rogers, M.D. The RIO 2 study will determine if the procedure, performed in an in-office setting, is as safe as procedures performed in a traditional hospital setting — such as an operating room, cardiac catheterization laboratory or electrophysiology laboratory.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
Medtronic plc announced that its Protege GPS self-expanding peripheral stent system has received approval from the U.S. Food and Drug Administration (FDA) for the treatment of stenotic lesions of the common and external iliac arteries.
Vital Images Inc. will debut its U.S. Food and Drug Administration (FDA) 510(k)-cleared CT Myocardial Perfusion application at the 10th annual scientific meeting of the Society of Cardiovascular Computed Tomography (SCCT). The meeting will be held in Las Vegas July 16-19.
Infinitt announced today that it has entered into a seven-year Partner Plus Agreement with TridentUSA Health Services based on an unlimited software licensing model for Infinitt PACS and Cardiology Suite, in addition to custom software development. The contract also encompasses Infinitt's embedded voice recognition and reporting software, as well as the new Gx Communicator package (Critical Results, Peer Review and Collaborator Instant Messaging).
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Konica Minolta Medical Imaging announced new Blue Moon Lifecycle Solutions designed to help customers minimize downtime, maximize productivity, and eliminate risk for the lifetime of their handheld Sonimage P3 and portable Sonimage HS1 ultrasound systems. The company will introduce the new Blue Moon Lifecycle Solutions at the Association for Medical Imaging Management (AHRA) annual meeting, July 20-22 in Las Vegas.
Thoratec announced that the HeartMate PHP (Percutaneous Heart Pump) received CE Mark approval, permitting sale in the European Union and other international countries. Approval was based on data from the first 30 patients enrolled in the HeartMate PHP SHIELD I CE Mark Trial examining use of PHP to support patients undergoing a high-risk percutaneous coronary intervention (PCI) procedure. Data from all 50 patients enrolled in this study will be presented later in 2015.
Biotronik announced the completion of the BIOVALVE first-in-human trial for its new transcatheter aortic valve. Study doctors successfully implanted the device in patients suffering from severe symptomatic aortic stenosis. The study, which established the transcatheter aortic valve implantation (TAVI) device's early safety at 30 days, was conducted at the University Heart Center Hamburg-Eppendorf (UKE), Germany.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
Mindray announced the release of its TE7 Touch Enabled Ultrasound System, which supports rapid and confident evaluation in multiple point-of-care (POC) settings. The intuitive tablet-like operation, high image quality with one-touch image optimization, and exam presets improve both diagnostic confidence and efficiency.
On July 8, the Centers for Medicare and Medicaid Services (CMS) released the first proposed update to the physician payment schedule since the repeal of the Sustainable Growth Rate in April. The proposal includes a number of provisions focused on person-centered care, and continues the Obama administration’s commitment to transform the Medicare program to a system based on quality and healthy outcomes.
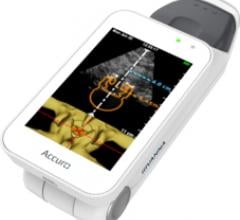
Rivanna Medical announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance to market Accuro, a handheld and untethered smart phone-sized ultrasound device. Accuro is designed to guide spinal anesthesia with automated 3-D navigation technology in addition to ultrasound imaging of abdominal, musculoskeletal, cardiac and peripheral vascular anatomies.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
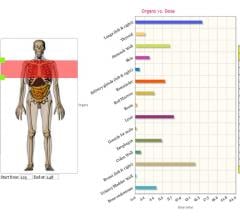
PHS Technologies Group LLC, a division of PACSHealth LLC, announced that it will integrate VirtualDose CT (computed tomography) software from Virtual Phantoms Inc. into their DoseMonitor product.

Hospitals performing carotid artery stenting vary considerably in rates of in-hospital stroke or death, according to a study published in JACC: Cardiovascular Interventions. Those rates can range from 0 to 18 percent overall, and from 1.2 to 4.7 percent when accounting for variation in health of patients at admission.

Edwards Lifesciences Corp. announced that it has agreed to acquire CardiAQ Valve Technologies Inc., a privately held company and developer of a transcatheter mitral valve replacement (TMVR) system.

 July 17, 2015
July 17, 2015