Critical Diagnostics announced it has expanded its cardiac biomarker testing product portfolio by CE marking the Aspect-PLUS ST2 test, a rapid quantitative ST2 test. Results are reported in under 30 minutes.
A team of scientists led by Johns Hopkins cardiologist and biomedical engineer Hiroshi Ashikaga, M.D., Ph.D., has developed a mathematical model to measure and digitally map the beat-sustaining electrical flow between heart cells.
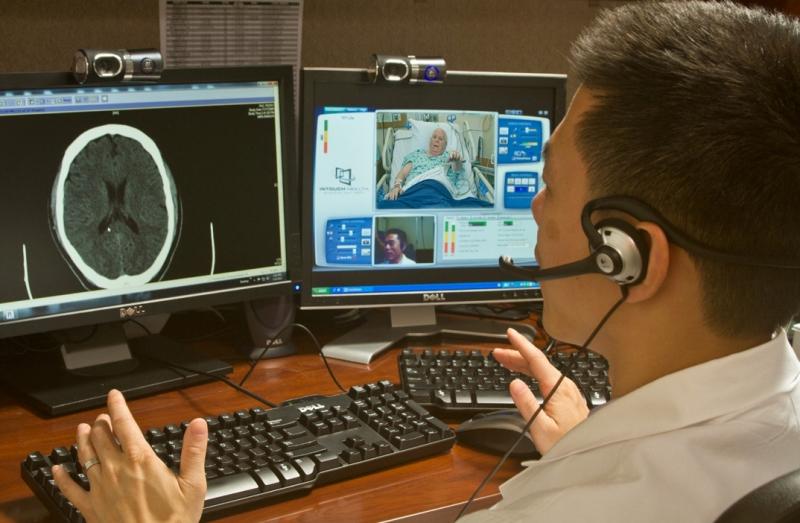
For the first time, researchers at The University of Texas Health Science Center at Houston (UTHealth) were able to enroll patients at other hospitals into an acute stroke clinical trial.
SPONSORED CONTENT — Studycast is a comprehensive imaging workflow system that allows healthcare professionals to work ...
The Society of Thoracic Surgeons (STS) has released the first publicly accessible national report of surgical outcomes from its Congenital Heart Surgery Database (CHSD), a component of the STS National Database. Public reporting results are available at www.sts.org/publicreporting.

Key data from many transcatheter aortic valve replacement (TAVR) clinical trials will be presented as late-breakers at the American College of Cardiology (ACC) Scientific Session this weekend, March 14-16, 2015, in San Diego, Calif. While the three-day conference will be packed with lectures, poster presentations, case studies, etc., most investors will be waiting for TAVR-related clinical data from Edwards and Medtronic. We expect these data to be positive for both vendors, which will only help to solidify their hold on the global TAVR market.
The first annual financial results of Xofigo (radium-223 dichloride) from Bayer are in line with the MEDraysintell analysis showing that the growth of nuclear medicine in the future will come through therapeutic radiopharmaceuticals (radiotherapeutics). Revenue for Xofigo – a product used in the treatment of prostate and bone cancers – reached EUR 157 million (US$ 209 million) in 2014.
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...
Researchers at the University of California, San Diego School of Medicine have identified a key piece in the complex molecular puzzle underlying heart failure.
Carroll Hospital Center physicians will now have 24/7 remote access to the University of Maryland Medical Center’s (UMMC) Brain Attack Team through a new telemedicine service for stroke patients.
George Adams, M.D., an interventional cardiologist with North Carolina Heart and Vascular, successfully treated a patient having an acute myocardial infarction (AMI) with the Aspire Mechanical Thrombectomy System.
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
In an analysis of outcomes of about 12,000 patients who underwent transcatheter aortic valve replacement (TAVR), death rate after one year was nearly one in four, according to a study in the March 10 issue of JAMA. Of those alive at 12 months, almost half had not been rehospitalized and approximately 25 percent had only one hospitalization.
Carestream is demonstrating its new Touch Ultrasound System at the American Institute of Ultrasound in Medicine (AIUM) annual meeting, March 21-25.
TeraRecon unveiled the latest release of the company’s flagship iNtuition enterprise viewing solutions in combination with the second-generation of its iNteract+ interoperability platform at the annual European Congress of Radiology (ECR) meeting, March 4-8 in Vienna.
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
NYU Langone Medical Center has announced the creation of a new multidisciplinary Venous Thromboembolic Disease Center (VTEC) to treat those with life-threatening blood clots. The new VTEC delivers advanced detection, comprehensive care and effective management for patients experiencing a venous thromboembolic event.
Nearly all women and people over 65 in the United States with atrial fibrillation are advised to take blood thinners under new guidelines based on an analysis from the Duke Clinical Research Institute (DCRI).
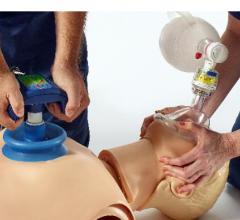
The U.S. Food and Drug Administration (FDA) approved the ResQCPR System, a system of two devices for first responders to use while performing cardiopulmonary resuscitation (CPR) on cardiac arrest patients. The devices may improve the patient’s chances of surviving cardiac arrest.

 March 11, 2015
March 11, 2015