There have been several recent advancements in implantable cardioverter defibrillator (ICD) technology to extend battery life, improvements in patient monitoring to avoid needless shocks, the introduction of quadripolar lead devices to improve device programming and to improve therapy effectiveness, and development of magnetic resonance imaging (MRI)-safe ICDs.
ICDs are implanted in patients who are at high risk for sudden cardiac arrest (SCA) due to sustained ventricular tachycardia or fibrillation. These devices also are used to improve the heart’s pumping ability in heart failure patients. ICDs are incorporated into cardiac resynchronization therapy defibrillator (CRT-D) devices, which resynchronize the contractions of both ventricles and have defibrillation capabilities. Several newer ICDs offer pacing functions, and many vendors offer wireless remote monitoring/interrogation of the device data with bedside sending units so patients do not need to come in for regular office visits.
Quadripolar Leads Expand Therapy Options
One of the biggest innovations has been the introduction of quadripolar lead devices. The leads use four electrodes to allow more programming options for pacing and overcoming issues with lead placement. This can help optimize CRT-D therapy and decrease the number of patients who do not respond. Up to 10 percent of heart failure patients' left ventricular (LV) lead placements are not successful because of anatomical challenges, phrenic nerve stimulation (PNS) or poor electrical measurement.
Use of a quadripolar LV lead instead of a bipolar option during CRT can decrease complications at six months, according to preliminary results presented at the 2014
European Society of Cardiology (ESC) Congress. The MORE-CRT trial demonstrated the safety and efficiency of the quadripolar lead technology in providing more options to manage CRT patients. The MORE-CRT study included 1,068 heart failure patients scheduled for CRT from 63 centers in 13 countries. The study includes more than 1,000 CRT patients with either a bipolar lead or a quadripolar lead. The study showed that at six months, compared to controls, patients with quadripolar leads were significantly more likely to be free from a composite endpoint of both intra- and post-operative LV lead-related complications (85.97 percent versus 76.86 percent, P=0.0001) — a relative risk reduction (RRR) of 40.8 percent. The driver of this benefit was mainly intra-operative complications, which were reduced by more than 50 percent in the quadripolar group (5.98 percent vs. 13.73 percent, P<0.0001, RRR 56.4 percent).
Additional data released in 2014 show use of quadripolar LV leads was associated with significantly reduced hospitalizations specific to heart failure and LV lead surgical revision. Presented at
Heart Rhythm 2014, The Hospitalization Rates and Associated Cost Analysis of Quadripolar versus Bipolar CRT-D: a comparative analysis of a single-center prospective Italian registry presentation, demonstrated quadripolar leads reduced the number of hospitalizations by 53 percent when compared to the non-quadripolar group. This hospitalization rate reduction translated into a statistically significant 62 percent reduction in overall costs for both healthcare systems and patients.
St. Jude Medical was the first to gain U.S. Food and Drug Administration (FDA) approval for a quadripolar pacing lead in January 2012, on its Unify Quadra CRT-D, which uses the Quartet left ventricular quadripolar pacing lead.
Boston Scientific received FDA approval in April 2014 for the Dynagen X4 and Inogen X4 CRT-Ds. The X4 line of quadripolar CRT-Ds offers 70 percent more pacing options to address high capture thresholds and PNS effectively, along with a large battery capacity and a six-year warranty.
Last August, Medtronic received FDA approval for the Attain Performa model 4298 quadripolar lead, and the Viva Quad XT and Viva Quad S CRT-D. The quadripolar lead and devices offer 16 pacing configurations and shorter spacing between the two center electrodes, and the Attain Performa model 4298 left-heart lead provides additional options for physicians to treat different patient anatomies. Medtronic launched two additional Attain Performa LV quadripolar leads in December with S-shape and straight leads to accommodate patients’ varying vessel sizes and curvatures to enhance successful lead placement.
Increasing ICD Battery Life
Battery longevity has long been an issue with ICDs. As patients live longer, they may need to undergo surgical procedures every few years for regular battery replacements. Increased device longevity can reduce the risk of infection and other complications due to repeat replacement procedures and help minimize out-of-pocket patient expenses for avoidable replacement procedures.
In February, Boston Scientific launched a line of extended longevity (EL) battery ICDs, including the Dynagen EL and Inogen EL device models. The devices use the EnduraLife battery, developed with high-performance chemistry and advanced manufacturing capabilities to provide up to double the battery capacity of other ICDs, the vendor said. Boston Scientific said the battery life is projected to be about 12 years before a replacement is needed. The EnduraLife battery is packaged in a device up to 11 percent smaller and 24 percent thinner than other ICDs.
"Battery longevity has a direct impact on patient outcomes and the cost of care," said
Samir Saba, M.D, who was the first to implant an EL ICD after FDA approval at The University of Pittsburgh Medical Center. "The EL ICD is an important advancement that can help minimize the frequency of avoidable replacement procedures to help reduce costs and the potential for replacement-related complications."
Subcutaneous ICD Technology
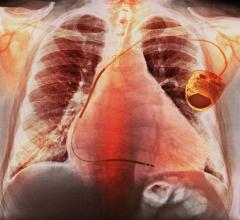
The FDA granted market clearance in October 2012 for Boston Scientifics’ S-ICD system, the world's first commercially available subcutaneous ICD (S-ICD). The S-ICD sits entirely just below the skin without the need for implantable leads to be placed inside the heart. This leaves the heart and blood vessels untouched, offering patients an alternative to transvenous ICDs, which require leads to be placed in the heart itself.
"The S-ICD System establishes the first new category of cardiac rhythm management devices since the introduction of cardiac resynchronization therapy," said
Raul Weiss, M.D., associate professor of clinical and cardiovascular medicine at The Ohio State University. "Doctors now have a breakthrough treatment option that provides protection from sudden cardiac arrest without touching the heart."
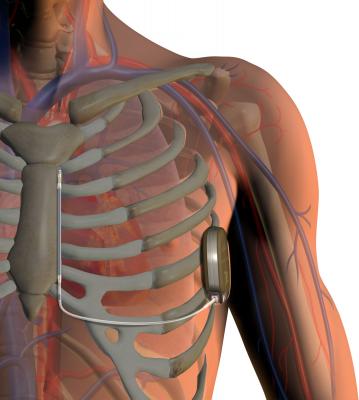
The S-ICD System is designed to provide the same protection from sudden cardiac arrest as transvenous ICDs. The system has two main components: (1) the pulse generator, which powers the system, monitors heart activity and delivers a shock if needed, and (2) the electrode, which enables the device to sense the cardiac rhythm and deliver shocks when necessary. Both components are implanted just under the skin — the generator at the side of the chest and the electrode beside the breastbone. Implantation with the S-ICD System is straightforward using anatomical landmarks, without the need for fluoroscopy, eliminating patient X-ray exposure.
External Defibrillator Offers Bridge Therapy
For patients at risk for SCA who are being evaluated for a permanent ICD, Zoll offers the LifeVest wearable external defibrillator as a temporary solution. The LifeVest allows a physician time to assess a patient’s long-term arrhythmic risk and make appropriate plans.
The LifeVest is lightweight and easy to wear under a patient's clothing, allowing them to return to their daily activities while having the peace of mind that they are protected from SCA. The LifeVest continuously monitors the patient’s heart and, if a life-threatening heart rhythm is detected, the device delivers a treatment shock to restore normal heart rhythm. This device continuously monitors the patient's heart with dry, non-adhesive sensing electrodes to detect life-threatening abnormal heart rhythms. If a life-threatening heart rhythm is detected, the device alerts bystanders and delivers a treatment shock to restore normal heart rhythm. The entire event, from detecting a life-threatening arrhythmia to automatically delivering a treatment shock, usually occurs in less than a minute.
The LifeVest is used for a range of patient conditions or situations, including following a heart attack and before or after bypass surgery or stent placement, as well as for those with cardiomyopathy or congestive heart failure that places them at particular risk. Zoll said the LifeVest has been prescribed to more than 100,000 patients.
Zoll intregrates this device with the LifeVest Network online patient data management system. It allows clinicians to monitor patient data online, downloaded from a patient’s LifeVest. It gives the clinician options to customize events that generate an alert, or to be notified for a number of events, such as detected arrhythmias, treatments, patient-recorded electrocardiograms (ECGs) and patient use.
MRI-safe ICDs
It is estimated that about 60 percent of ICD patients will need an MRI within 10 years of receiving a device. Until the availability of MR-conditional ICD systems, patients with devices have been contraindicated from receiving MRI scans because of potential interactions between the MRI and device function. There are several MRI-compatible ICDs now available in Europe and two undergoing FDA investigational device exemption (IDE) pivotal trials in the United States
The ProMRI trial is evaluating Biotronik’s DX ICD system, which gained CE mark in Europe in 2011, becoming the first MRI-compatible ICD on the market. In May 2014, Medtronic announced the first U.S. trial implant of its Evera MRI SureScan ICD system. Medtronic gained European CE mark approval for the device in April 2014.
Reducing Shocks
Patients’ quality of life and faith in their ICDs can be significantly decreased if their ICD shocks the patient needlessly. Some vendors now include technology to help reduce inappropriate shocks.
St. Jude Medical's Assura ICD and CRT portfolio features Secure Sense RV Lead Noise Discrimination, an algorithm that expands St. Jude’s ShockGuard technology and offers advanced sensing options designed to reduce the incidence of inappropriate shocks. The algorithm provides advanced alerts as well as more proactively lowering the risk of lead-related complications through its ability to automatically withhold tachycardia therapy in the presence of lead noise (over-sensing of electrical signals). The technology differentiates lead noise from true ventricular tachycardia (VT) or ventricular fibrillation (VF) episodes that require life-saving therapy.
In addition, ShockGuard technology features specific programming that distinguishes between rhythms that require defibrillation therapy and those that do not, such as benign arrhythmias. DecisionTx programming offers advanced sensing technology designed to avoid sensing unwanted signals (T-waves) and more anti-tachycardia pacing options, which can convert many fast ventricular arrhythmias painlessly and avoid the need for high-voltage shocks.
Medtronic’s Evera MRI includes SmartShock 2.0, a shock reduction algorithm that enables the device to better differentiate between dangerous and harmless heart rhythms. Medtronic said studies estimate about 20 percent of patients with implantable defibrillators might experience inappropriate shocks in response to a benign arrhythmia or electrical noise sensed by the device.
Lead failure is another source of inappropriate shocks. In late 2013, Medtronic gained FDA clearance for its Lead Integrity Alert (LIA) software for use with non-Medtronic leads. LIA can report performance issues on Durata and Riata (St. Jude Medical) and Endotak (Boston Scientific) defibrillator leads when connected to a Medtronic device.
Reducing Implanted Hardware
Biotronik’s Ilesto family of ICD/CRT-D devices includes the Ilesto DX. The platform allows for a 15 percent size reduction and 29 percent fewer components with no compromise in longevity or clinical features. It is the first defibrillator system equipped to provide full atrial diagnostic information with just one specialized defibrillator lead. The DX system offers an alternative with more monitoring capability than a single-chamber device, and without additional leads required for dual-chamber devices.





 February 02, 2024
February 02, 2024