May 21, 2015 — Important new data presented during the Heart Rhythm Society's (HRS) 36th annual Scientific Session supporting improved outcomes and cost-effectiveness of the CardioMEMS HF System for the management of Class III heart failure patients.
A presentation during HRS 2015 included “Pulmonary Artery Pressure Management in Heart Failure Patients with Cardiac Resynchronization Therapy or Implantable Cardioverter Defibrillator Devices Significantly Reduces Hospitalizations and Mortality Above and Beyond Background Guideline-Directed Medical Therapy.” These data were presented by William Abraham, M.D., co-primary investigator of the landmark CHAMPION clinical trial, which supported FDA approval of the CardioMEMS HF System.
The retrospective analysis showed that heart failure (HF) management using pulmonary artery (PA) pressure monitoring improved outcomes in patients already receiving optimal treatment including both medical management with Guideline Directed Medical Therapy (GDMT) and device therapy with an implantable rhythm device, such as an implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy (CRT) device.
“Even in patients being managed in accordance with American College of Cardiology and American Heart Association guidelines for medical management and device therapy, we saw a decrease in all-cause hospitalizations and mortality for Class III heart failure patients when we added pulmonary artery pressure monitoring with the CardioMEMS HF System,” said Abraham, chief of cardiovascular medicine at The Ohio State University Wexner Medical Center. “These data show a 43 percent reduction in heart failure hospitalizations and 53 percent reduction in mortality, which demonstrates the added value of PA pressure monitoring in an already well managed patient population.”
Cost Effectiveness of CardioMEMS
A new economic analysis of data from the CHAMPION trial showed an Incremental Cost-Effectiveness Ratio (ICER) based on all-cause comprehensive management of heart failure patients of $30,167 per quality adjusted life year (QALY) gained when the patients were managed by PA pressure monitoring. The ICER is a statistic used to summarize the cost-effectiveness of a healthcare intervention. It is defined by the difference in cost between two possible therapeutic interventions, divided by the difference in their effect on quality of life and survival. The analysis “Cost Effectiveness Assessment of Pulmonary Artery Pressure Monitoring for Heart Failure Management”“ was presented by Philip Adamson, M.D., co-primary investigator of trial.
“These data, presented at HRS, show that the economic value for pulmonary artery pressure monitoring is well below the U.S. acceptable cost effectiveness threshold,” said Adamson, who is medical director and vice president of medical affairs for St. Jude Medical. “We are encouraged by these analyses indicating that the CardioMEMS HF System is a cost-effective solution.
Additional CHAMPION trial data analysis presented at HRS 2015 by Lee Goldberg, M.D., Hospital of The University of Pennsylvania, evaluated patients from the CHAMPION trial and found that heart failure hospitalizations were reduced the most when PA pressures from the CardioMEMS HF System were used rather than clinicians relying on clinical signs and symptoms alone. These findings show that early intervention through the identification of increased PA pressure weeks prior to clinical signs and symptoms, significantly reduce HF hospitalizations. In addition, previously presented data at the American Heart Association (AHA) 2014 meeting showed a 58 percent reduction in 30-day all-cause readmissions when using the CardioMEMS HF System, which can minimize a hospital’s risk of Medicare penalties due to high-readmission rates.
CardioMEMS HF System
The CardioMEMS HF System is supported by strong clinical evidence, including data from the CHAMPION (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Patients) trial published in The Lancet. The randomized, controlled CHAMPION clinical trial proved the effectiveness of the CardioMEMS HF System in New York Heart Association (NYHA) Functional Classification System class III HF patients who had been hospitalized for HF in the previous 12 months. In contrast to large-scale studies of tele-monitoring of weight, blood pressure and transthoracic impedance such as Tele-HF, TIM-HF and DOT-HF, the CHAMPION study showed that management based on PA pressures led to a clinically significant reduction in HF admissions. Specifically, the trial demonstrated a statistically and clinically significant 28 percent reduction in the rate of HF hospitalizations at six months, and 37 percent reduction in HF hospitalizations during an average follow-up duration of 15 months.
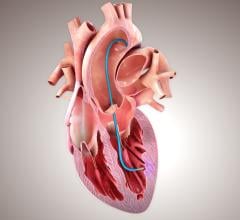
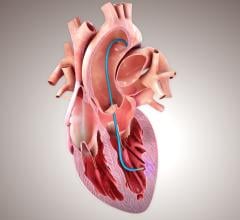
The CardioMEMS system uses a miniaturized, wireless monitoring sensor that is implanted in the PA during a minimally invasive procedure to directly measure PA pressure. Measuring PA pressure allows clinicians to proactively manage treatment with medication changes for patients with worsening HF before visible symptoms, such as weight and blood pressure changes, occur. The system allows patients to transmit PA pressure data from their homes to their health care providers, who then manage appropriate medication changes to reduce the likelihood of hospitalization.
The Center for Disease Control and Prevention reports that more than 5 million Americans suffer from HF with 670,000 new cases diagnosed each year. Roughly 1.4 million patients in the United States. have NYHA Class III HF, and historically these patients account for nearly half of all HF hospitalizations. According to the American Heart Association, the estimated direct and indirect cost of HF in the United States for 2012 was $31 billion and that number is expected to more than double by 2030.
Heart failure occurs when the heart is unable to pump enough blood to meet the body’s demands and blood pressure within the heart is elevated. Significant HF progression over a period of days is known as acute decompensation and leads to hospitalization. Increased PA pressures often precede indirect measures of worsening HF such as weight and blood pressure changes. The CardioMEMS HF System, approved by the U.S. Food and Drug Administration in May 2014, allows clinicians to stabilize PA pressures by proactively managing medications and other treatment options while also providing an early indication of worsening HF.
For more information: sjm.com


 April 16, 2024
April 16, 2024