
The Biosense Webster multi-electrode balloon RF ablation catheter. Each electrode can have varied power settings to avoid damage to underlying tissues like the esophagus.
Electrophysiology (EP) technology has been advancing rapidly the past few years with new ablation tools to improve atrial fibrillation (AF) treatments, miniaturized diagnostic monitoring systems, and new implantable rhythm management devices that are making procedures much less invasive.
To see examples of some of the technologies explained below, watch the VIDEO “Editor's Choice of Most Innovative New Technology at HRS 2017.”
Leadless Pacemakers
One of the biggest issues with implantable EP devices is the leads that connect the device to the heart. Leads are frequently cited as the weakest component of pacing, implantable cardioverter defibrillator (ICD) or cardiac resynchronization therapy (CRT) due to wearing out or complications due to infection. Traditional implantable devices also require surgery to install the leads and the can, increasing the complexity of the procedure, adding cost and exposing the patient to infection risks. Wireless technologies and the miniaturization of both electronic components and the batteries have enabled the development of transcatheter implantable pacing and now CRT systems, eliminating the need for surgery or the placement of venous leads.
In early 2016, the U.S. Food and Drug Administration (FDA) approved the Medtronic Micra device, the first leadless, catheter-implanted pacemaker approved in the United States. It is the world’s smallest pacemaker at 0.8 cc in size, being a little smaller than its competitor, the Abbott/St. Jude Medical Nanostim. The device has a 20 French diameter and uses a 27 French introducer in the femoral vein, allowing catheter access to the right ventricle. Micra has four self-expanding nitinol hooks that extend as it is unsheathed from its delivery catheter. These act as an anchor, hooking into the trabeculation at the apex of the right ventricle. The operator performs a tug on it to ensure it will not embolize prior to final release. Out of more than 1,600 patients in the post-FDA approval study of the device, there has only been one embolization of a Micra. The battery, while small, is expected to have a 12-year life.
The Abbott/St.Jude Medical Nanostim pacemaker is currently pending final FDA review. The single chamber pacemaker device is designed to be fully retrievable. It has a docking button on the top of the device which can be grasped by a snare catheter and twisted to turn the device and unscrew the corkscrew-like anchor in the myocardium.
There were recent battery issues with the Nanostim device, causing its distribution in Europe, where it is currently approved, to be paused in 2016. St. Jude said it has now updated the battery technology. The battery life is expected to be nine to 10 years, depending on the pacing requirements for the patient.
It is an 18 French device that is delivered via catheter directly into the apex of the right ventricle. Vascular access is gained through the femoral vein and device guidance is done under angiography.
One issue with these two leadless pacing systems is that they are single-chamber devices, which only account for about 10-15 percent of the U.S. pacemaker market. Medtronic said it views the Micra system as a stepping-stone platform to the next generation of cardiac pacing. This may include the implantation of a Micra pacemaker in each ventricle of the heart and enabling wireless communications between the devices to synchronize their pacing.
“The problem with regular pacemakers is the wire that goes to the heart, because as the heart is beating and the wire has all this motion, over the course of time you can hear breaks in the wires. The idea with a leadless pacemaker is that you take the whole wire out of the equation,” said Vivek Reddy, M.D., director of cardiac arrhythmia services and professor of medicine, cardiology, Mount Sinai Hospital, N.Y.
Reddy said as a single-chamber pacing system, both the Micra and the Nanostim perform very well according to clinical trial data, and Medicare is now reimbursing use of these devices. He said the main limitation to wider adoption is the single-chamber pacing. “Today, the main limitation with these devices is that we do not have the possibility of doing atrial pacing, and most importantly dual-chamber pacing. These devices are just the first step and the companies are working on ways to do atrial/ventricular pacing,” he explained. “Ultimately, the goal is to avoid the use of a lead, which has always been the weak link in pacemaker systems.”
Wireless CRT
CRT systems typically use an epicardial coronary sinus pacing lead for the left ventricle (LV), but placement of the lead on the outside of the heart is not ideal because of issues with the coronary sinus anatomy or scar tissue. This may be the cause of about 30 percent of patients not responding well to CRT therapy. Placing the lead inside the LV is ideal, but not practical with current technology.
EBR Systems is developing the WiSE CRT system, the first endocardial, leadless CRT pacing system. It uses an electrode about the size of a large grain of rice that is implanted inside the wall of the LV using transcatheter delivery. A wireless ultrasound transducer is surgically implanted between the ribs to send ultrasound energy to the electrode, which converts the waves into electrical energy for pacing, eliminating the need for a battery or lead wire, allowing the device to be very small. This works as an adjunct device to work in combination with an existing connected pacemaker, ICD or CRT device. The conventional system senses the RV pacing and can work with the WiSE system to synchronize the LV.
“There is a study we did in Europe using this where we showed the technology does work. It improves heart failure in patients who failed biventricular pacing, it improved ejection fraction, functional class and everything else we looked at,” Reddy said. “This technology is going to be studied very soon in a large multi-center randomized trial here in the United States.”
EBR Systems has CE mark approval for the system in Europe, where there are about 100 patients implanted with the WiSE system. The company is working to initiate an FDA investigational device exemption (IDE) trial in the U.S.
Improved EP Ablation Technologies
Intracardiac ablation systems can cure or improve cardiac function due to arrhythmias by killing tracts of heart tissue to block the pathways of faulty electrical signals. This is most often done using pulmonary vein isolation (PVI). However, this technology requires EPs to literally connect the dots if using traditional point-by-point ablation catheters. This can be difficult, even for the most experienced operators, inside the moving heart using the surrogate visualization provided by electro-mapping systems. The difficulty is illustrated in the fact that atrial fibrillation (AF) ablations are unsuccessful in about 40 percent of patients. Ablation procedures are also very lengthy, often lasting several hours.
“Ablation therapy is growing at an extremely rapid rate, but it is still not a perfect procedure,” said Hugh Calkins, M.D., FACC, FAHA, FHRS, director of cardiac arrhythmia services and professor of medicine at Johns Hopkins Hospital. “Part of that is because we don’t have a perfect understanding of the mechanisms of atrial fibrillation, and part of that is because we don’t have perfect tools to accomplish what we want to accomplish.”
Calkins said about 70-80 percent of AF patients who go back for a repeat ablation procedure have at least one PVI that failed and reconnected to continue the arrhythmia. He said there is a lot of room for improvement with the technology and there are several new techniques being developed.
He said Medtronic’s cryoballoon ablation system has seen rapid, widespread adoption in the past year since the release of positive data from the FIRE AND ICE trial. The study showed better outcomes with the cryoballoon compared to radiofrequency (RF) ablation. An economic analysis from that trial also savings from fewer rehospitalizations and repeat ablations.
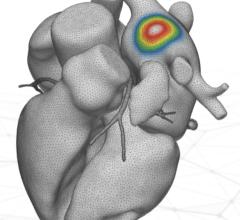
HeartLight was granted FDA clearance in 2016 for its laser ablation balloon technology indicated for pulmonary vein isolation to treat AF. The system consists of a compliant balloon that seats in the ostia of the pulmonary veins and a laser inside the catheter can be rotated around to ablate the tissue. It also has a camera inside the catheter to offer direct visualization of the ablation and location of the laser, eliminating the need for electro-mapping systems and cutting procedural time. The lesions are created with 20-30 second ablations. About 25 ablations are needed to isolate a pulmonary vein with lesion overlap. The combination of the balloon, camera and variable-energy, steerable ablation is believed to be able to eliminate the interoperator variability in ablation procedures. Another advantage of the system is that it can deliver variable energy, so the energy of the laser can be dialed down when ablating near the esophagus or other neighboring critical structures.
A late-breaking HRS trial highlighted a first-in-human study for the Biosense Webster RF balloon catheter in treating patients with AF. The 39-patient RADIANCE study showed it could uniformly achieve pulmonary vein isolation (PVI) in all patients without the need for “touch-up” with a focal ablation catheter. The system uses a balloon that is lined with several electrodes. The energy level for each electrode can be tailored to prevent damage to neighboring nerves or the esophagus.
Calkins said another technology to watch is the development of noninvasive focused ultrasound to perform PVI ablations without the need for catheterization.
Increasing Safety in EP Lead Extractions
One of the biggest safety concerns in removing old device leads is the possibility of tearing the superior vena cava (SVC). This requires immediate emergency surgical repair to stop the bleeding and the complication currently has a 50 percent mortality rate. However, Spectranetics Bridge Occlusion Balloon, introduced in 2016, offers a new safety net during procedures, allowing rapid inflation of an intravascular balloon to seal the tear and allow the surgical team time to prep and perform a repair without fear of the patient bleeding out. The device is credited with saving about 20 lives in the past year since gaining market clearance.
EP lead management expert Bruce Wilkoff, M.D., Cleveland Clinic, said the device is one of the most important new developments in lead extraction technology and has become part of Cleveland Clinic’s lead extraction protocol. He said the balloon offers a safety net to minimize the effect of a potentially catastrophic SVC tear. While rare, he said this complication has made some physicians shy away from referring patients for lead extractions in the past.
Watch the VIDEO "EP Lead Extraction Strategies," an interview with Bruce Wilkoff, M.D., at HRS 2017.
Subcutaneous ICDs
Boston Scientific introduced the first subcutaneous implantable cardioverter defibrillator (S-ICD) system in 2009. Since then, there have been many studies published showing the system delivers very effective therapy and reduces the invasiveness of traditional ICD implants by eliminating the leads to the heart. Instead, the system uses a lead placed under the skin of the chest over the heart, eliminating transvenous leads or the need for lead management later on.
“The leads are the Achilles heel of both pacemakers and ICDs, so these new platforms take the leads out of the vasculature,” said Lucas Boersma, M.D., Ph.D., FESC, St. Antonius Ziekenhuis, Nieuwegein, The Netherlands, who was heavily involved in the S-ICD trials. However, he said one drawback of the S-ICD is it cannot deliver pacing, so it limits its patient population. “Many patients still need pacing, and right now it is still not available, so the future direction is to come up with hardware solutions that can pace.”
He said this may include incorporating a separate pacemaker device in combination with the S-ICD. Boersma explained Medtronic is developing substernal ICD leads below the ribs so they are closer to the heart, but not inside vessels. This will include a pacing electrode. He also mentioned the EBR WiSE wireless CRT technology as another future direction.
About 40 percent of the ICD market today is composed of cardiac resynchronization therapy (CRT) systems with pacing. Of the remaining 60 percent, Boersma said between 30-50 percent would benefit from S-ICD therapy if they do not have any need for pacing.
A new technology in development is the “string ICD,” which replaces the can and cardiac leads with a long, thin cable-like device that is implanted subcutaneously in the patient's chest. The first-of-its-kind NewPace Ltd. ISSD string ICD was developed to eliminate the need for an active generator and places only a small, flexible device including coils below and over the ribs and totally subcutaneously. It only requires two very small incisions with no need to create a pulse generator pocket. This results in minimal anatomical protrusion, for improved patient comfort and aesthetic appearance. First-in-man data for 22 patients implanted with the ISSD was presented at HRS showed an average implant time of 20 minutes.
Watch the VIDEO “Overview of Subcutaneous ICD Technology,” an interview with Lucas Boersma, M.D., at HRS 2017.
Replacing Holters With Wearable and Implantable Devices
Both the American College of Cardiology (ACC) and HRS meetings have seen an explosion of vendors with simple, small, wearable, stick-on Holter monitoring systems that eliminate the need for a bulky, belt-worn device and the placement of multiple wire leads on the patients. These new devices offer a less expensive, even disposable option to traditional, durable Holter monitoring systems. Some vendors offer the devices themselves, others offer the devices in connection with monitoring services.
Another monitoring technology that is seeing increasing usage are implantable cardiac monitors (ICMs). Biotronik, Medtronic and St. Jude Medical offer different iterations of these monitors, which are placed subcutaneously in the chest using a simple, fast, in-office procedure. The Medtronic Linq device is credited by many with pushing the technology to the next level, because of its small size (about a quarter of the size of a USB thumb drive) which can easily be inserted under the patient’s skin.
Abbott/St. Jude Medical received CE mark in May 2017 for its latest version, the Confirm RX. It pushes remote electrocardiogram (ECG) monitoring to the next level as the first implantable EP device to interface with the patient’s smartphone. The phone, usually carried on the patient, acts as the wireless monitoring base unit to receive data from the device and enable transmission to a monitoring service or a physician’s office.
Data from the HRS 2017 late-breaking REVEAL AF study showed ICMs used for long-term, 24-hour-a-day monitoring, detected a high incidence of AF in patients previously undiagnosed but suspected to be at high-risk for AF and stroke. The study found that at 18 months, continuous monitoring with either the Medtronic Reveal XT or the Reveal Linq resulted in an AF detection rate of 29.3 percent among previously undiagnosed high-risk patients. The data showed continuous monitoring with an ICM detected AF beyond 18 months with a detection rate of 40 percent at 30 months. Additionally, 6.2 percent of patients were diagnosed with AF at 30 days, indicating that more than three-quarters of high-risk patients with AF would have gone undetected with only 30 days of cardiac monitoring. The median time from device insertion to the first AF episode was 123 days, which is outside the range of conventional external monitoring.
New Electro-mapping Systems
For years the EP mapping system market was dominated by Biosense Webster and St. Jude Medical, but there were complaints from EPs that there was little progress in advancing the technology to overcome some of its limitations. This changed in 2014, when Boston Scientific launched the Rhythmia mapping system. At HRS 2017, the updated Rhythmia HDx was launched. The system can show lower voltages than on previous-generation mapping systems. It uses a 64-electrode basket catheter to create very detailed, high-density electro-maps with as many as 50,000 to 60,000 points.
“All the mapping systems are getting better, and the introduction of the Rhythmia super-high density mapping system has really put pressure on the other companies to come up with their own really-high-density mapping systems,” Calkins said. “Clearly, the whole field is moving toward more accurate mapping, more points, clearer electrograms, and hopefully this will translate into better efficacy with outpatients.”
Biosense Webster is now developing a new mapping system. In December 2016, Abbott/St. Jude Medical received FDA clearance for the Ensite Precision system. It was designed to improve the reliability and accuracy for AF ablation procedures. It includes the launch of the Advisor mapping catheter, which incorporates magnetic sensing technology. This works with a magnetic sensor placed under the patient to more accurately locate mapping points and the tip of the catheter inside the anatomy. It also helps increase the accuracy of the anatomical model of the heart the system creates to guide procedures. St. Jude said this has eliminate distortion of the model that was a drawback of the previous-generation system.
A new technology that may offer a big reduction in mapping/procedural times is the Acutus Medical AcQMap High Resolution Imaging and Mapping System. It uses a basket catheter with 48 electrodes combined with 48 tiny ultrasound transducers. The basket can be manually rotated around inside the atrium to rapidly “paint” a very accurate combined electro- and anatomical map simultaneously in about five minutes. Conventional EP mapping systems can take 20 minutes or longer to complete the mapping process. The electrodes do not need to contact the walls of the heart because the vendor said they can detect the electrical field created by cardiac contractions. The system was approved in Europe in 2016 and the company said it will soon be submitting for FDA 510(k) approval.
MRI To Guide Ablation Procedures
iMRIcore is redesigning devices to make them safe for use in a magnetic resonance imaging (MRI) environment to perform MRI-guided ablation procedures. MRI offers many advantages over X-ray angiography and traditional mapping systems. It can image without any radiation, eliminating the need to wear heavy protective aprons. Second, unlike angiography, it can image soft tissue and visualize the tissue response to ablations and when a scar forms, allowing EPs to see the pattern and effectiveness of their ablation points and patterns.
The catheters iMRIcore developed have MRI receiver coils near the tip so they clearly show up on live MR imaging.
The company has European CE mark approval for its EP recorder/stimulator system, but its catheters are still pending market clearance and they hope for approval by the end of 2017 so they can start a commercial launch in 2018. The company has seven EP lab installs in Europe that were used for the CE mark trials. It is working with a U.S. university medical center to begin early feasibility studies for an eventual launch in the U.S. market. The vendor says it has been approached by a handful of luminary U.S. heart centers that are looking at building out new EP labs using MRI instead of angiography.
New EP Leads Introduced
The FDA recently granted market approval for the Biotronik Ilivia CRT system and two new CRT and ICD leads. The Plexa ProMRI shocking lead utilizes a unique spiral-wound segment of wire to help reinforce the segment of the lead where it beds as it enters the heart and tends to be a fatigue point with leads due to constant flexing from cardiac motion. The Sentus ProMRI lead is the first quadripole lead introduced by Biotronik and is the thinnest quadripolar left ventricular lead available in the United States.
Ultrasound Guidance in the EP Lab
Two vendors have developed lightweight, compact, cart-based ultrasound systems that allow high-quality intracardiac echocardiography (ICE) and transesophageal echo (TEE) in the EP or cath lab. Both systems offer many of the features of premium echocardiography systems.
Siemens Healthineers released its new Acuson P500 ICE Edition compact ultrasound system at HRS 2017. It offers ICE to help guide transeptal punctures and has a transducer to aid in needle guidance or vascular access. The system also has an interface with BioSense Webster’s mapping system. It does not have TEE capability, but does offer transthoracic echo (TTE). It offers color and spectral Doppler capabilities as well.
GE Healthcare displayed its Vivid iq compact system, which weighs 4.5 kg and is built for tough conditions and for mobile use in the cath and EP lab. It matches the high image quality and high-end functionality of GE's premium systems, including 3-D/4-D TEE. It is intuitive to use with a touchscreen and requiring fewer keystrokes.
Watch the VIDEO “Advances in Electrophysiology Technology,” a discussion with Heart Rhythm Society (HRS) President Michael Gold, M.D., Ph.D., director of cardiology and associate dean at the Medical University of South Carolina (MUSC), at the American College of Cardiology 2017 annual meeting.
Read the related article New Technology and Market Challenges Facing Implantable Cardioverter Defibrillators




 September 21, 2023
September 21, 2023 








