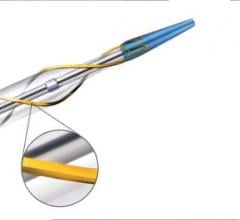
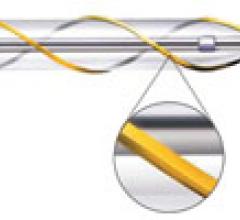
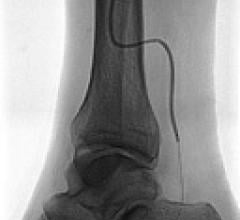
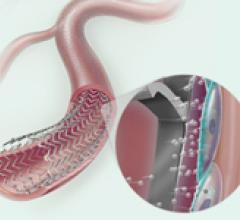
In recent years, many medical device companies saw the underserved lower extremity as an opportunity to enter the ...
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
April 28, 2010 – Deviating from its focus on intravascular imaging systems and fractional flow reserve (FFR) ...
March 26, 2010 – The FDA granted 510(k) market clearance for the AngioSculpt PTA Scoring Balloon Catheter for ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
February 22, 2010 – As part of a comarketing and trademark license agreement between MEDRAD Inc. and B. Braun ...
January 25, 2010 – Expanding its peripheral vascular disease product offerings, Medtronic Inc. today signed an ...
January 20, 2010 – The balloon material of the new REEF HP PTA Balloon Catheter was created for use in peripheral ...
November 18, 2009 – As part of a new partnership deal with QXMédical LLC, W. L. Gore & Associates will distribute ...
October 21, 2009 – Cook Medical is introducing six new products in parallel to provide better treatment options to ...
October 1, 2009 – Invatec recently launched the REEF HP PTA Balloon Catheter at CIRSE 2009, which the company said ...
September 21, 2009 — Minnow Medical recently announced it will be presenting interim clinical data regarding a ...
The FoxCross PTA Catheter is a next-generation .035 balloon dilatation catheter is used to open peripheral ...
July 16, 2009 – Cook Medical received clearance today from the FDA to market its newest balloon dilatation ...
July 14, 2009 – Cardiovascular Systems Inc. (CSI) said yesterday the first patient has been enrolled in its ...
July 2, 2009 – Researchers from Dartmouth-Hitchock Medical Center (DHMC) in Lebanon, N.H., have published a new ...
The focus on balloon angioplasty largely slipped from the interventional limelight a decade ago after the ...


 May 02, 2010
May 02, 2010