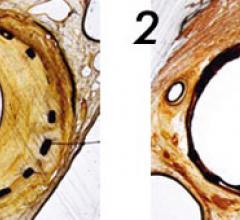
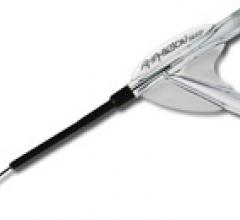
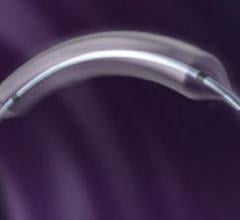
June 9, 2009 - AngioScore Inc. said today its flagship product, the AngioSculpt Scoring Balloon Catheter, received ...
Balloon Catheter
This channel includes news and new technology innovations for angioplasty balloon catheters (PTA). These are used in arteries with atherosclerotic lesions to compress the plaque expand the artery lumen to reopen occluded or heavily stenosed atherosclerotic lesions. Balloons are often used in combination with a stent to prop the treated vessel segment open. In addition to plain old balloon angioplasty (POBA), this section includes news about drug-coated balloon (DCB), valvuloplasty balloons and specialty cutting balloon.
May 19, 2009 - Invatec announced today the availability of its newly CE-marked coronary balloon, the IN.PACT ...
May 10, 2009 - Medtronic Inc. Friday announced the settlement of all royalty disputes with Johnson and Johnson (J ...
While the current positive revolution in percutaneous coronary intervention (PCI) practice has been made possible by the ...
April 14, 2009 - The voluntary medical device recall initiated on Feb. 2, 2009 by Arrow International’s Cardiac ...
April 2, 2009 – Invatec received 510(k) clearance from the FDA to market its Amphirion Deep 150 mm Long PTA ...
March 16, 2009 – Invatec today announced CE-certification of a new coronary balloon, the IN.PACT Falcon paclitaxel ...
March 10, 2009 - Abbott today launched the VOYAGER NC Coronary Dilatation Catheter, a next-generation balloon ...
VOYAGER NC is Abbott's next-generation balloon dilatation catheter for the treatment of coronary artery disease ...
February 2, 2009 - Cook Medical’s new Advance PTX drug-eluting balloon was used in a live case transmitted from ...
January 21, 2009 - Invatec this week announced the European launch of a new peripheral balloon, the IN.PACT Amphirion ...
January 13, 2009 - Micell Technologies, a development-stage biomedical device company dedicated to developing ...
December 10, 2008 – ev3 today said it received FDA 510(k) clearance from the FDA to market its EverCross 0.035-inch and ...
December 4, 2008 - Medtronic Inc. recently began its U.S. launch of the Sprinter Legend (semicompliant) and the ...
November 13, 2008 - Medtronic Inc. today started its U.S. market launch of the Endeavor Sprint drug-eluting stent ...
November 10, 2008 - Boston Scientific Corp. today said it received FDA approval to market its Apex PTCA ...


 June 09, 2009
June 09, 2009