May 14, 2014 — Data presented during Heart Rhythm 2014 found the use of quadripolar leads reduced the number of ...
Cardiac Resynchronization Therapy Devices (CRT)
Cardiac Resynchronization Therapy Devices (CRT) are cardiac electrophysiology (EP) systems that include a small electronic device that is surgically implanted into a pocket of skin to help both ventricles contract together. They are also called biventricular pacemakers. These systems can be used for pacing only, or can include a built-in defibrillator.
April 17, 2014 — Medtronic announced the U.S. Food and Drug Administration (FDA) approved an expanded indication for ...
By Dave Fornell
The biggest news item from the American College of Cardiology (ACC) 63rd Annual Scientific Session ...

By Dave Fornell, DAIC editor
Data from several cutting-edge device clinical trials will be presented as late-breakers ...
March 21, 2014 — Boston Scientific Corp. received CE marking and launched in Europe the Ingevity family of magnetic ...
October 14, 2013 — An independent study from the University of Pittsburgh Medical Center, published online this week in ...
October 1, 2013 — The U.S. Food and Drug Administration (FDA) approved Biotronik’s Ilesto family of implantable ...
September 30, 2013 — Medtronic Inc. announced clinical trial results showing that heart failure patients treated with ...
September 13, 2013 – The U.S. Food and Drug Administration (FDA) Circulatory System Devices Panel of the Medical Devices ...
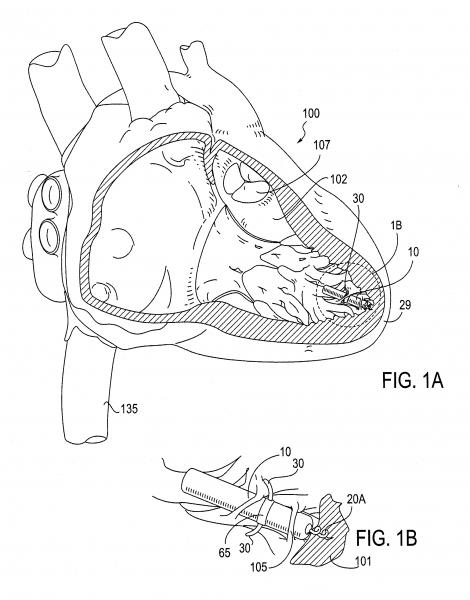
July 25, 2013 — The 2013 ESC (European Society of Cardiology) Guidelines on Cardiac Pacing and Cardiac Resynchronization ...
June 28, 2013 — St. Jude Medical Inc. announced CE mark approval of its next-generation quadripolar device, the Quadra ...


 May 14, 2014
May 14, 2014