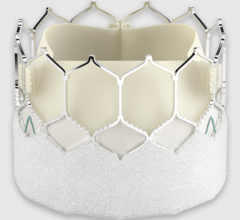
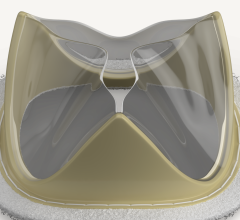
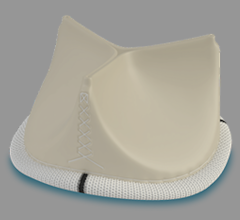
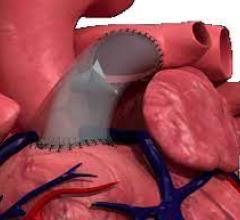
December 18, 2023 — Death rates related to infective endocarditis declined in most adults across the U.S. within the ...
Heart Valve Technology
This channel includes news and new device innovations about heart valve technologies, including the aortic valve, mitral valve, pulmonic valve, and tricuspid valve. This includes information on transcatheter valve technologies like transcatheter aortic valve replacement (TAVR, or implantation TAVI), transcatheter mitral valve repair or replacement (TMVR), transcatheter and surgical valve repairs, and surgical replacement valves. Newer devices are now being used for transcatheter tricuspid valve repair replacement (TTVR).
December 12, 2023 — Patients who received the anticoagulant drug warfarin after bioprosthetic aortic valve replacement h ...
November 20, 2023 — Abbott announced new late-breaking data that show advanced heart failure patients living with its He ...
November 20, 2023 — Student engineers from the University of Bath are on top of the world after winning an international ...
November 10, 2023 — BiVACOR, a clinical-stage medical device company developing the Total Artificial Heart (TAH), has ...
October 26, 2023 — The Society of Thoracic Surgeons and the European Association for Cardiothoracic Surgeons have issued ...
October 19, 2023 — Edwards Lifesciences Corporation announced the company's EVOQUE tricuspid valve replacement system ...
October 17, 2023 — The Patel Children's Heart Institute at St. Joseph's Children's Hospital achieved a milestone ...
October 5, 2023 — A newly-issued "Scientific Statement on Durable Mechanical Circulatory Support" in the October issue ...
September 20, 2023 — The Minneapolis Heart Institute Foundation (MHIF), an internationally renowned cardiovascular ...
June 13, 2023 — PECA Labs, a medical device company reimagining vascular grafts and valves with durable polymeric ...

 December 18, 2023
December 18, 2023