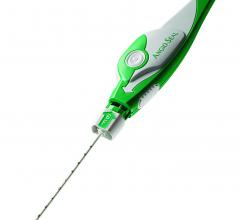
February 1, 2019 — Merit Medical Systems Inc. announced that the PreludeSync Distal Compression Device is now available ...
Hemostasis Management
This channel includes news and new technology innovations for devices to help stop bleeding at vascular access sites. These include coated or treated bandages to accelorate or augment the clotting cascade, vascular closure devices and compression devices, including radial artery compression systems.
December 20, 2018 — Cardiva Medical Inc. announced the company has received premarket approval (PMA) from the U.S. Food ...
November 21, 2018 – Vivasure Medical Ltd. recently announced the European launch of the PerQseal closure device for ...
Philippe Genereux, M.D., co-director of the structural heart program at the Gagnon Cardiovascular Institute at ...
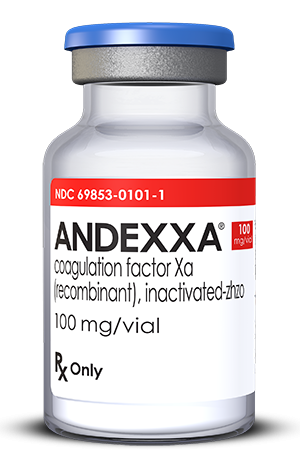
May 7, 2018 — The U.S. Food and Drug Administration (FDA) has approved Portola Pharmaceuticals' Andexxa, the first ...
April 10, 2018 – Cardiva Medical announced the company received U.S. Food and Drug Administration (FDA) approval for an ...
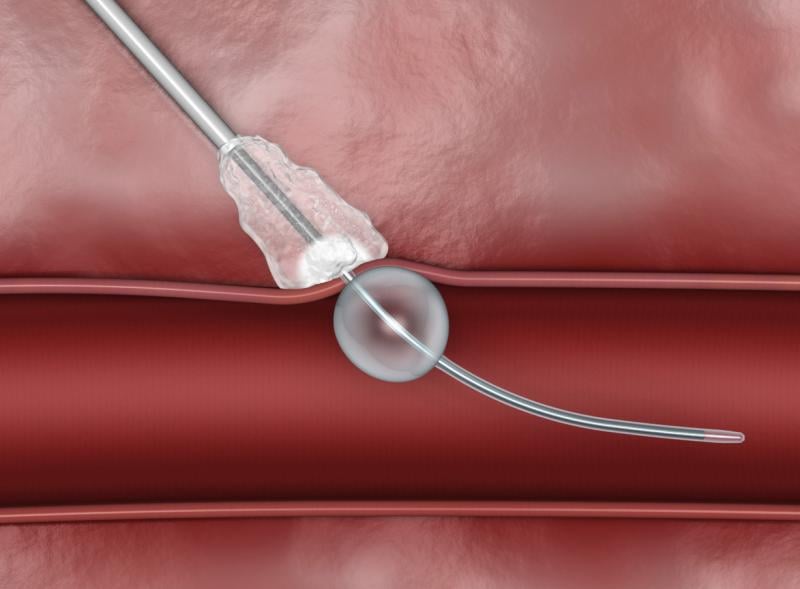
While manual compression remains the gold standard for hemostasis of catheterization vascular access site arteriotomies ...
October 18, 2016 — Abbott and St. Jude Medical Inc. announced today an agreement to sell certain products from their ...
September 12, 2016 — Vivasure Medical announced last week that the company has completed a Series C financing of €16.2M ...
August 24, 2016 — Whether severe trauma occurs on the battlefield or the highway, saving lives often comes down to stopp ...
August 3, 2016 — The U.S. Food and Drug Administration (FDA) has granted market clearance to Rex Medical’s bioresorbable ...
January 18, 2016 — Vivasure Medical announced Conformité Européenne (CE) Mark approval of the world’s first fully ...
January 6, 2016 — The Medicines Company announced Dec. 18 it has entered into a purchase agreement pursuant to which ...
December 16, 2015 — Scientists at CBSET have presented preclinical evidence that the Manta Large Bore Vascular Closure ...


 February 01, 2019
February 01, 2019