Leads Implantable Devices
News and new technology innovations for leads implantable devices can be found on this channel.


June 6, 2013 — Data presented at Heart Rhythm 2013 shows that Medtronic Inc. Lead Integrity Alert (LIA) software ...
May 13, 2013 — The Population Health Research Institute (PHRI) has conducted a further independent analysis of data ...
May 6, 2013 — St. Jude Medical announced its first enrollment in its MultiPoint Pacing clinical study to build upon its ...
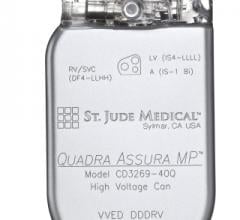
April 30, 2013 — St. Jude Medical Inc. announced CE Mark approval and European launch of its Allure Quadra Cardiac ...
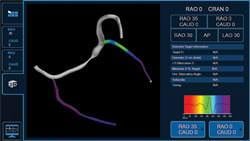
April 10, 2013 — Medtronic Inc. announced market release of the CardioGuide Implant System, a novel real-time navigation ...
March 18, 2013 — Medtronic Inc. announced it has received CE mark and will begin the European launch of the Attain ...
February 25, 2013 — The U.S. Food and Drug Administration (FDA) granted final approval for the Biotronik Lumax 740 DX ...
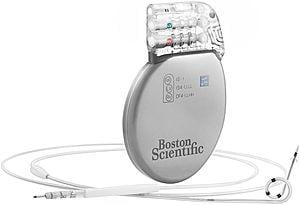
December 28, 2012 — The first patient has been implanted with the Boston Scientific Corporation next generation Ingevity ...
December 21, 2012 — Nanostim Inc. announced the first successful implants of a leadless pacemaker in a series of 11 ...
December 18, 2012 — Zoll Medical Corp. announced that its new U.S. Food and Drug Administration (FDA)-cleared OneStep ...
October 29, 2012 — Boston Scientific Corporation has received regulatory approval to market the Reliance 4-Front lead ...
October 8, 2012 — The U.S. Food and Drug Administration (FDA) has granted Boston Scientific Corp. regulatory approval ...

 January 23, 2014
January 23, 2014