Renal Denervation
This channel includes news and new technology innovations for renal denervation used to treat hypertension. Renal denervation is a catheter-based therapy. The catheter is into the renal artery and delivers radio-frequency energy to ablate the nerves in the vessel wall that regulate vasoconstriction. This leaves the vessel permanently in the widest open position to allow greater blood flow to the kidneys. This enables greater filtration of the blood and removal of excess water that causes hypertension.

December 6, 2013 – Medtronic, Inc. presented three-year data from SYMPLICITY HTN-2, the first and longest-running ...
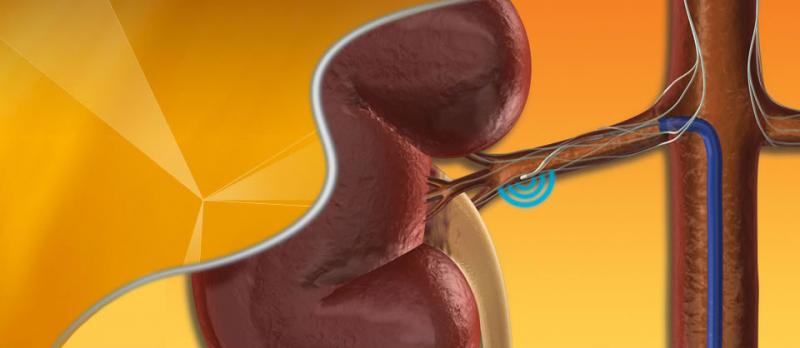

October 14, 2013 — Since its debut in 2010, the renal denervation market has generated enthusiasm across the medical ...
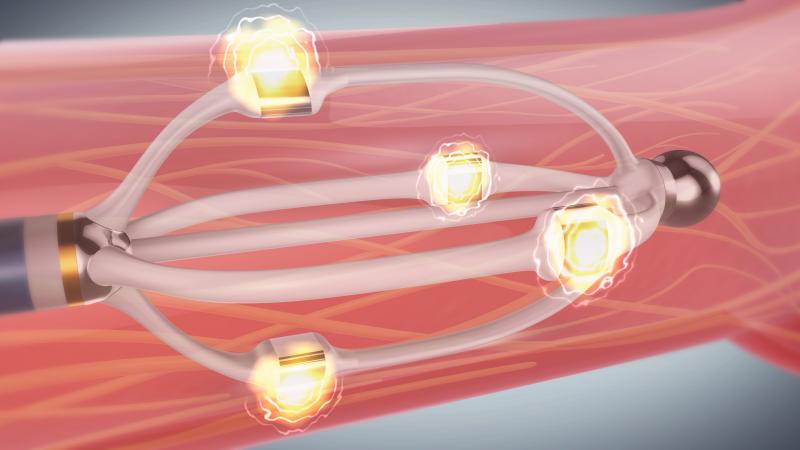
September 3, 2013 — St. Jude Medical Inc. announced the CE mark approval of its next-generation EnligHTN renal ...
August 22, 2013 — Qinghui Chen, assistant professor in Michigan Tech’s kinesiology and integrative physiology department ...
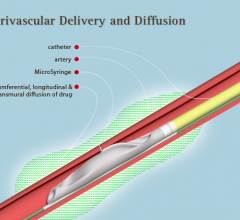
August 2, 2013 — Mercator MedSystems has been issued U.S. Patent 8,465,752, which protects the company’s sole U.S ...
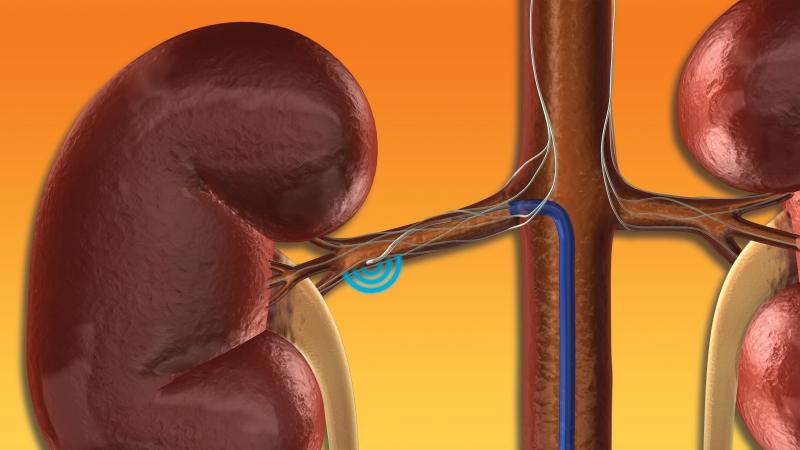
One of the exciting new frontiers for interventional cardiology is the use of renal denervation therapy to treat ...
The field of interventional cardiology is one of constant change and innovation. The past 10 years have documented ...
June 13, 2013 — St. Jude Medical Inc. announced U.S. Food and Drug Administration (FDA) approval to begin the EnligHTN ...

 December 24, 2013
December 24, 2013