November 12, 2018 — The Vascade MVP vascular closure system met its endpoints compared to manual compression in a pivotal clinical study for multi-access venous closure following electrophysiology (EP) procedures, according to manufacturer Cardiva Medical Inc. The AMBULATE study is the first randomized pivotal trial of vessel closure conducted exclusively by EP physicians.
These positive results were presented in the Late-Breaking Presentations section of the American Heart Association’s (AHA) Scientific Sessions, Nov. 10-12 in Chicago by Andrea Natale, M.D., executive medical director, Texas Cardiac Arrhythmia Institute.
“The feedback from our patients and staff was extremely positive with Vascade MVP during the study,“ said Natale. “Patients with previous procedures noticed a significant improvement in their level of comfort due to the much shorter bed rest time, and the time saved overall enabled us to implement a new more efficient workflow in the hospital. Additionally, there was a substantial decrease in the use of post-procedural pain medications in patients receiving the Vascade device. This type of innovation will be particularly important as demand for these procedures continues to grow.”
The AMBULATE study was a randomized, controlled clinical trial that enrolled 204 patients who underwent arrhythmia ablation procedures by 28 physicians at 13 sites across the United States. All patients had multiple (3 or 4) mid-bore (6-12 Fr inner diameter sheath) femoral venous access sites, and were randomized into one of two groups. The treatment group had all sites closed with the Vascade MVP System, while the control group had all sites closed using manual compression, which is the current standard of care. Patients were enrolled with both radiofrequency and cryo energy sources used in their procedures. Principal investigators included Natale; Mintu Turakhia, M.D., associate professor, Stanford University School of Medicine; and Steve Compton, M.D., Alaska Heart and Vascular Institute.
Compared to manual compression, the Vascade MVP System met all primary and key secondary endpoints, including:
- Time to Ambulation (primary endpoint, a measure of how quickly patients are able to get up on their feet and walk following vessel closure). The Vascade MVP arm showed a median reduction of 3.9 hours (2.2 hours vs. 6.1 hours, p-value < 0.0001);
- Total Post-Procedure Time. The Vascade MVP arm showed a mean reduction of 3.7 hours in the total time from completion of the ablation procedure to the patient walking. This was inclusive of the time to achieve vessel closure (3.1 hours vs. 6.8 hours, p-value <0.0001);
- Time to Discharge Eligibility. Vascade MVP reduced the mean time for patients to be deemed eligible for discharge by 3.4 hours (3.1 hours vs. 6.5 hours, p-value < 0.0001).
- Patient Satisfaction. The patient-reported level of satisfaction with the duration of bed rest was 63 percent higher in the Vascade MVP arm (8.3 score out of 10 vs. 5.1 out of 10, p-value <0.0001); and
- Opioid Pain Medications (ad hoc analysis). Fifty-eight (58) percent fewer patients in the Vascade MVP arm used opioid-class pain medications following their procedure (15 percent of patients vs. 36 percent of patients, p-value 0.001).
Additional studies in the AMBULATE series include:
- AMBULATE IMPACT Study – a recently completed study that examines today’s workflow following ablation procedures with a focus on improving hospital economics through early patient ambulation and improved workflow. Results from the AMBULATE IMPACT study will be published in 2019.
- AMBULATE CAP Study – a continued access protocol study designed to evaluate the safety of Vascade MVP with earlier hospital discharge, elimination of urinary catheters and elimination of protamine drug use in the setting of electrophysiology procedures. The AMBULATE CAP protocol is currently being conducted with approval of the U.S. Food and Drug Administration (FDA).
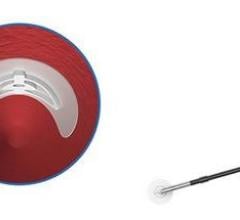
Vascade MVP is a fully integrated, extravascular, bioabsorbable femoral access closure system that is easy to use and leaves no permanent components behind. The system combines Cardiva’s collapsible disc technology and a thrombogenic resorbable collagen patch in an integrated design.
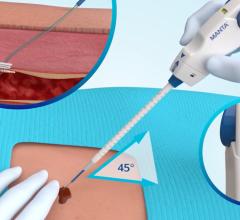
The device works by placing a small, collapsible mesh disc against the inside of the vessel wall to temporarily stop the bleeding, releasing a collagen patch into the tissue and then removing the mesh disc. The collagen patch expands, providing a mechanical and physiological seal to stop the bleeding, and then absorbs into the body, leaving nothing behind and allowing further access to the vessel if additional procedures are required.
For more information: www.cardivamedical.com


 April 16, 2024
April 16, 2024