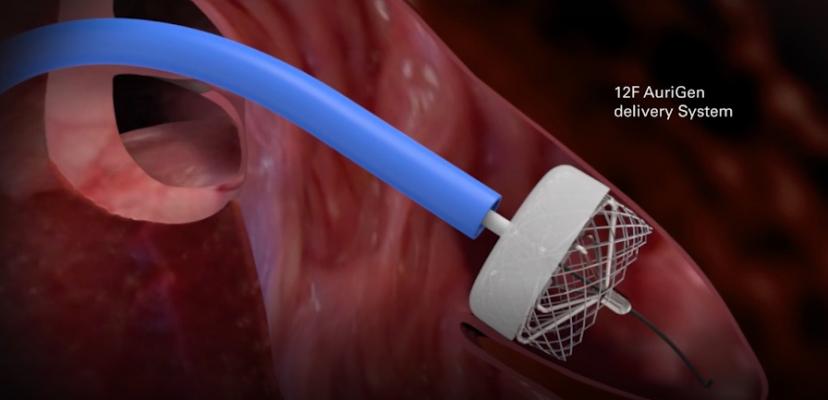
December 18, 2018 — Irish medical device company AuriGen Medical won the prestigious Global Innovation Award at the International Conference for Innovations in Cardiovascular Systems (ICI), Dec. 2-4 in Tel Aviv, Israel. AuriGen Medical, which specializes in the treatment of atrial fibrillation, is developing the first heart implant to treat both the stroke and heart failure risk associated with this often fatal condition.The Global Innovation award attracted entries from the best medical device and pharmaceutical start-ups from around the world.
Everyday over 30 million patients suffer with atrial fibrillation, an irregular heartbeat which can lead to stroke, dementia, heart failure and death. Unfortunately for the majority of atrial fibrillation patients with persistent longstanding disease, the current treatment options are routinely ineffective, which results in billions in direct healthcare costs.
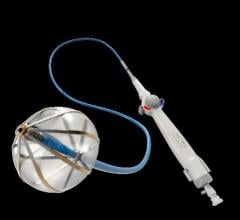
The AuriGen Device incorporates patented sensor and implant technology. This technology is designed to quickly and safely isolate the source of atrial fibrillation and filter blood clots, preventing them from transferring to the brain, thereby reducing the risk of stroke.
AuriGen Medical recently ranked first out of almost 1,300 EU Horizon 2020 funding applications. The technology has secured a €2.5 million cash injection from the European Union's €80 billion research and innovation program.
For more information: www.aurigenmedical.com


 March 28, 2024
March 28, 2024