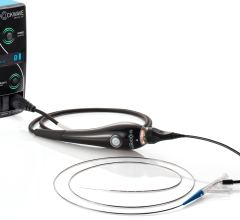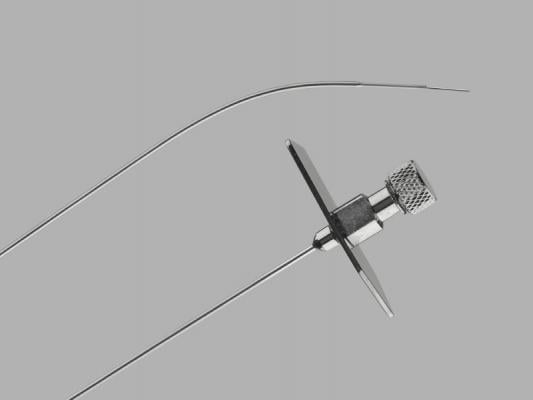
March 20, 2019 — Cook Medical is recalling one lot of its Transseptal Needle due to a manufacturing error that resulted in some needle tips missing the back bevel that creates a sharp tip. Without a back bevel, the needle tip could damage the inside of the introducer sheath during insertion of the needle, resulting in detached plastic fragments. These fragments could potentially enter the patient’s bloodstream and result in serious adverse health consequences such as a longer procedure to retrieve the plastic pieces, injury to blood vessel walls, embolism, stroke or death.
The Transseptal Needle is used by surgeons to access the left side of a patient’s heart during cardiac procedures. Fluoroscopy medical imaging and a guide catheter help the surgeon place the needle in the heart.
On Feb. 1, 2019, Cook Inc. sent an Urgent Medical Device Recall notification letter to affected customers. The notice asked customers to:
-
Share notice with appropriate staff;
-
Identify and remove any affected transseptal needles and quarantine affected needles;
-
Immediately stop all distribution and use of affected products;
-
Return any affected product(s) to Cook Inc. Medical with a copy of the Acknowledgement and Receipt Form to receive a product credit; and
-
Complete and return the Acknowledgement and Receipt Form by fax at 812.339.7316 or email at [email protected]
The recalled products are in lot number 8833687, model TSNC-18-71.0. Impacted devices were manufactured on April 23, 2018, and distributed between May 30, 2018, and Nov. 5, 2018. A total of 97 devices are affected by the recall in the U.S.
Healthcare professionals and distributors with questions are instructed to contact Cook Medical Customer Relations by phone at 800-457-4500 or 812-339-2235, Monday through Friday between 7:30 a.m. and 5:00 p.m. Eastern Time, or by email to [email protected].
For more information: www.cookmedical.com


 December 20, 2023
December 20, 2023