July 25, 2008 - CryoCath Technologies Inc. said this week results previously presented from three European study centers using the company’s Arctic Front, a cryotherapy product to treat cardiac arrhythmias, were published in the Journal of the American College of Cardiology (JACC).
The peer-reviewed article entitled, “Circumferential Pulmonary Vein Isolation with the Cryoballoon Technique,” appears in the July 22 edition of JACC. It discusses the three-center trial in which 74 percent of the 293 paroxysmal AFib patients enrolled were AFib free and off anti-arrhythmic drugs (AADs) after only one procedure with Arctic Front upon a median follow-up time of 12 months.
The study, conducted in Germany, was led by Dr. Vogt from the Herz und Diabetes Zentrum NRW in Bad Oeynhausen, Prof. Dr. Schumacher from Herz- und Gefäss-Klinik in Bad Neustadt and Dr. Pitschner from the Kerckhoff-Klinik in Bad Nauheim.
The patients enrolled in the 346-person study were predominantly paroxysmal AFib sufferers (n=293) with a small group of persistent AFib patients (n=53). Of the 1,403 pulmonary veins treated, 1,360 (or 97 percent) were ablated with Arctic Front or Arctic Front in combination with CryoCath’s Freezor MAX catheter demonstrating the broad applicability of Arctic Front cryoablation. The median number of applications per vein was 2.8 and the median total procedure time was 170 minutes confirming the ease of use and speed with which physicians are able to use Arctic Front compared with other ablation technologies.
The safety data reported in the article confirms the strong safety profile Arctic Front has demonstrated to date, the company said. There were no observed reports of atrio esophageal fistula, stroke, death or peri-interventional complications. Phrenic nerve palsies were reported in 7.5 percent of patients treated; in some instances these were resolved prior to the end of the procedure or hospital discharge and all cases were resolved by the 12-month follow up visit.
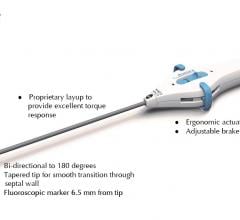
Arctic Front is a minimally invasive cryo-balloon catheter designed specifically to treat paroxysmal Atrial Fibrillation. This bi-directional, double balloon catheter enables physicians to rapidly isolate all four pulmonary veins for the treatment of AFib. More than 2,600 patients have been treated in more than 45 centers across Europe.
For more information: http://content.onlinejacc.org, www.cryocath.com


 April 03, 2024
April 03, 2024