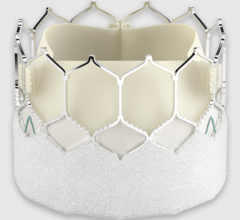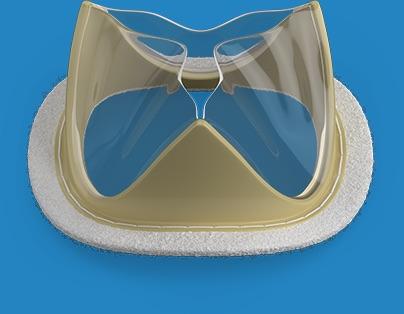
February 18, 2019 — Foldax Inc. announced the U.S. Food and Drug Administration (FDA) has granted investigative device exemption (IDE) approval for an Early Feasibility Study of the Tria surgical aortic heart valve to treat aortic valve disease.
Using LifePolymer, a proprietary advanced biopolymer material, and a proprietary valve design, the Tria heart valve is designed to be a lifetime valve with hemodynamic performance and quality of life similar to natural human valves but without using animal tissue or the side effects of long-term anticoagulants. Intended for use in transcatheter or surgical heart valve applications, the Tria heart valves are robotically manufactured to provide the highest level of quality and precision.
“We look forward to introducing the Tria heart valve to patients in the United States”, said Amit Patel, M.D., of the University of Utah, who will be serving as primary investigator in upcoming clinical trials. “With the Tria heart valve we have the opportunity to overcome the challenges with today’s heart valves. The Tria valve is truly a revolutionary option for the more than 1 million Americans who have moderate or severe heart diseases.”1
The complete Tria heart valve platform will include valves developed for use in aortic, mitral and tricuspid valve disease. The company plans to continue its development efforts and clinical trials, in pursuit of outside-the-U.S. (OUS) and U.S. regulatory approvals.
For more information: www.foldax.com
Foldax Gets $20 Million to Finance Innovative Heart Valve Technology
References
1. Heart Valve Society of America (2018, April 11). About the Heart Valve Society of America. Retrieved from http://www.heartvalvesocietyofamerica.org/more.html.


 April 17, 2024
April 17, 2024