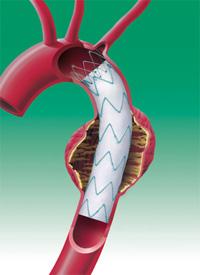
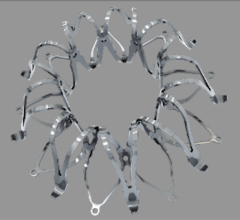
The first patient has been enrolled in the Medtronic Dissection Trial, which evaluates the clinical performance of the Valiant Thoracic Stent Graft with the Captivia Delivery System for the treatment of acute, complicated Type B aortic dissection, a serious cardiovascular condition associated with high morbidity and mortality.
Medtronic, Inc., in order to expand the use of minimally invasive endovascular procedures, initiated its Dissection Trial in May 2010 and will enroll a total of 50 patients across 25 centers in the United States. The study is being conducted under an Investigational Device Exemption (IDE) in the United States.
Dr. Zvonimir Krajcer, M.D., co-director of the Peripheral Vascular Disease Service at St. Luke’s Episcopal Hospital in Houston, treated the trial’s first patient with Type B aortic dissection.
“Patients with acute, complicated Type B aortic dissection require immediate treatment, and the Valiant Captivia system holds great promise as a minimally invasive treatment for this challenging patient group,” said Dr. Krajcer. “This trial will help to determine if the Valiant Captivia system is a safe and effective alternative to invasive surgery for these patients.”
Aortic dissections are classified as Type A or Type B depending on where they occur. Type A aortic dissections begin in the ascending aorta, the segment closest to the heart, and require surgery to repair. Type B aortic dissections begin in the descending aorta, may extend into the abdomen and, if uncomplicated by rupture or malperfusion, can be treated with medication as a first-line intervention.
Patients with acute, complicated Type B aortic dissections are reported to have a greater than 50 percent likelihood of dying from this disease and as such often require emergent treatment.
Type B aortic dissections have historically been treated with medication or through invasive surgical techniques.
Endovascular stent grafting is an effective way to treat some aortic conditions, such as aneurysms (a bulge in the wall of the aorta). Once deployed, the grafts conform to the wall of the aorta, the body’s main artery, creating a new path for blood flow.
The Valiant Thoracic Stent Graft received the CE Mark in 2005. Indicated for the treatment of a variety of thoracic aortic lesions, the device has been used to treat more than 15,000 patients worldwide.
Both the Valiant Thoracic Stent Graft and the Captivia Delivery System are investigational in the United States, where their use is limited to clinical trials approved by the U.S. Food and Drug Administration.
Medtronic is committed to advancing the treatment of cardiovascular disease through collaboration with leading clinicians, researchers and scientists worldwide.
For more information: www.medtronic.com

 April 22, 2024
April 22, 2024