April 8, 2013 — W. L. Gore & Associates Inc. has received U.S. Food and Drug Administraion (FDA) approval for the new large diameter 35 mm trunk-ipsilateral leg and 36 mm aortic extender components, as well as the lower profile 31 mm diameter trunk-ipsilateral leg and 32 mm aortic extender components of the Gore Excluder AAA Endoprosthesis. The new components provide physicians with a proven and durable endovascular option to treat abdominal aortic aneurysms (AAAs).
“The expanded sizing options benefit patients and physicians alike. The low profile sheath allows physicians to use the Gore Excluder device on aortic necks measuring up to 32 mm, providing more patients with access to minimally invasive endovascular treatment options using a superior device,” said Scott L. Stevens, M.D., professor, Department of Surgery, University of Tennessee Medical Center.
Compatible with an 18 French Gore DrySeal Sheath, the new 35 mm trunk-ipsilateral leg and 36 mm aortic extender represent some of the lowest profiles for treating infrarenal aortic necks measuring up to 32 mm in diameter, expanding the overall treatment range to 19-32 mm. The lower profile 31 mm diameter trunk-ipsilateral leg and 32 mm aortic extender components allow the trunk-ipsilateral leg component to be used with an 18 French Gore DrySeal Sheath and the 32 mm aortic extender component to be used with a 17 French compatible sheath to treat aortic necks with an inner diameter of 27-29 mm.
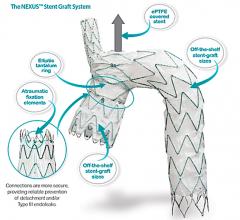
No changes have been made to the Gore Excluder device itself — instead, Gore has implemented an innovative process using ePTFE materials to constrain the device onto the catheter. This key enhancement to the simple delivery of the device reduces the access vessel requirement (6.8 mm) for patients requiring minimally invasive endovascular AAA repair. As with all of the lower profile devices, the 35 mm trunk-ipsilateral leg endoprosthesis and the 31 mm trunk-ipsilateral leg endoprosthesis are only available with the Gore C3 Delivery System.
“While endovascular repair of AAAs has proven to be a successful alternative to open surgical repair, physicians continue to seek improvements to the procedures,” said Fred Weaver, M.D., Professor of Surgery at the Keck School of Medicine of the University of Southern California. “This new lower profile 31 mm trunk-ipsilateral leg will expand the treatment options for more patients while continuing to provide flexibility and long-term conformability.”
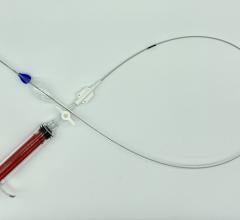
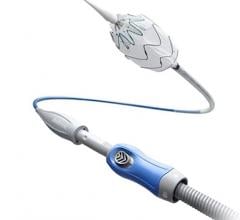
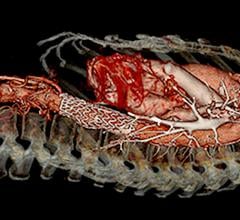
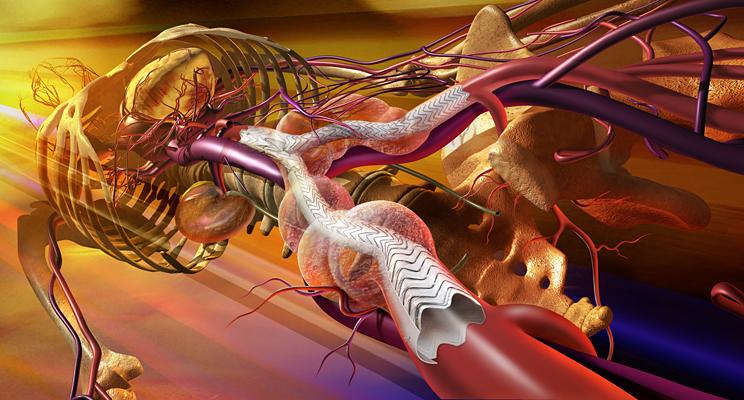
The Gore Excluder AAA Endoprosthesis is an endovascular stent-graft that seals off the aneurysm and creates a new path for blood flow. The device is inserted through a small incision in the patient’s leg using a catheter-based delivery technique. Once the physician has positioned the graft in the diseased aorta, the Gore C3 Delivery System uniquely and intuitively enables repositioning of the stent-graft. The ability to reposition the device may minimize complications that could occur if the graft needs to be moved after the initial deployment.
“Expanding our AAA sizing portfolio means that Gore can now offer physicians a wider range of components using the preferred infrarenal placement on the lowest profile sheath available today,” said Ryan Takeuchi, Gore Aortic business leader. “All of our innovative product enhancements, such as the ones announced today, are driven by Gore’s commitment to help physicians provide the best possible patient care.”
The Gore Excluder AAA Endoprosthesis is the result of collaboration between leading endovascular aneurysm repair (EVAR) physicians and Gore engineers. With more than 159,000 devices distributed worldwide, the Gore Excluder device is supported by a highly rated clinical support team, a comprehensive educational offering and Gore’s outstanding community awareness programs.
For more information: www.goremedical.com

 October 30, 2023
October 30, 2023