October 20, 2021 — HeartFlow Inc. announced the commencement of the REVEALPLAQUE (A pRospEctiVe, multicEnter study to AnaLyze PLAQUE using CCTA) trial to evaluate HeartFlow’s non-invasive plaque technology, an automated, deep learning-based method for identifying, characterizing and segmenting plaque in the coronary arteries. The first two patients were enrolled at Bryan Heart in Lincoln, Neb., by Brock Cookman, D.O., MSA, FACC, FSCAI, interventional cardiologist, and Clyde R. Meckel, M.D., FACC, FSCAI, interventional cardiologist.
Heart attacks are the most common cause of death for both men and women, yet 4 out of 5 deaths are preventable with lifestyle and nutrition changes if patients who are at high risk of a heart attack are identified early.[1] Understanding plaque burden – where plaque is in the coronary arteries, the amount of plaque and the type of plaque – is one of the best ways for physicians to identify patients at high risk of death from a heart attack.[2]
“For most patients, heart attacks happen without any warning symptoms. It is clear, however, that coronary plaque is the driving force behind understanding a patient’s risk of having a heart attack,” said Meckel, lead principal investigator for REVEALPLAQUE at Bryan Heart. “The tools available today for understanding plaque burden tend to be cumbersome or provide inconsistent results. To be able to accurately and non-invasively understand a patient’s plaque burden would be game changing in physicians’ abilities to save a patient’s life from a heart attack.”
The REVEALPLAQUE trial is planned to enroll 250 patients with stable coronary artery disease from approximately 15 sites across the U.S. and Japan. The primary endpoint of the trial is the level of agreement across total plaque volume and the characteristics of plaque as measured by the non-invasive HeartFlow plaque technology compared to intravascular ultrasound (IVUS) imaging, which is considered the standard for obtaining information about plaque burden.[3,4] Other endpoints to be measured include percent plaque volume, calcified plaque volume, low attenuation plaque volume, and fibrous plaque volume.
The U.S. lead principal investigators of the trial are Thomas Stuckey, M.D., Cone Health, North Carolina, and Jagat Narula, M.D., Mount Sinai Hospital, New York; and the Japan lead principal investigator is Dr. Gaku Nakazawa, Kindai University, Japan.
“We’ve known for years that plaque burden matters and CT angiography for assessing plaque burden is now advanced enough that it can be applied to many more patients across the spectrum of coronary disease. Combining the power of CT angiography and HeartFlow’s deep learning technologies has the potential to be a powerful tool in the physician’s arsenal of tests for assessing heart health,” said Campbell Rogers, M.D., FACC, chief medical officer, HeartFlow. “HeartFlow is committed to leveraging our core technology to provide innovative solutions that can help providers gain insights about anatomy, physiology and plaque to enable them to provide precise diagnoses and deliver optimal treatment plans for their patients.”
The REVEALPLAQUE study builds upon HeartFlow’s EMERALD (Exploring the MEchanism of Plaque Rupture in Acute Coronary Syndrome Using Coronary CT Angiography and ComputationaL Fluid Dynamics) and EMERALD II trials. In the EMERALD trial, the HeartFlow technology incorporating both plaque features and physiology was able to accurately predict the high-risk plaques that were most likely to rupture and cause a heart attack. The EMERALD II trial is currently enrolling patients.
The HeartFlow plaque technology is an automated method which analyzes coronary computed tomography (CT) scans and will enable clinicians to visualize, characterize and quantify plaque. This information could enable longitudinal assessment of coronary artery disease (CAD) to understand a patient’s response to medical therapy and lifestyle modifications over time. The plaque technology is based on HeartFlow’s proprietary deep learning-enabled algorithms, which have been trained on more than 15 million coronary CT images. HeartFlow’s plaque technology is currently available for investigational use only and is not available for commercial use.
About the HeartFlow FFRct Analysis
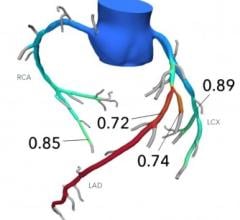
Starting with a standard coronary computed tomography angiogram (CTA), the HeartFlow Analysis leverages algorithms trained using deep learning (a form of AI) and highly trained analysts to create a digital, personalized 3D model of the heart. The HeartFlow Analysis then uses powerful computer algorithms to solve millions of complex equations to simulate blood flow and provides FFRct values along the coronary arteries. This information helps physicians evaluate the impact a blockage may be having on blood flow and determine the optimal course of treatment for each patient. A positive FFRct value (≤0.80) indicates that a coronary blockage is impeding blood flow to the heart muscle to a degree which may warrant invasive management.
Data demonstrating the safety, efficacy and cost-effectiveness of the HeartFlow Analysis have been published in more than 425 peer-reviewed publications, including long-term data out to five years. The HeartFlow Analysis offers the highest diagnostic performance available from a non-invasive test.[5] To date, clinicians around the world have used the HeartFlow Analysis for more than 100,000 patients to aid in the diagnosis of heart disease.
For more information: www.heartflow.com
References:
1. https://www.cdc.gov/vitalsigns/million-hearts/index.html
5. Driessen, R., et al. Comparison of Coronary Computed Tomography Angiography, Fractional Flow Reserve, and Perfusion Imaging for Ischemia Diagnosis. JACC. 2019;73(2),161-73.


 December 20, 2023
December 20, 2023