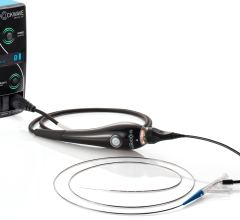
February 13, 2019 — Le Bonheur Children's Hospital cardiologists in Memphis, Tenn., implanted the Amplatzer Piccolo Occluder, the world's first commercially-approved medical device for transcatheter patent ductus arteriosus (PDA) closure in babies weighing as little as two pounds. The self-expanding, wire mesh device developed by Abbott is inserted through a small incision in the leg and guided through vessels to the heart, where it is placed to seal the opening of the heart.
Shyam Sathanandam, M.D., medical director of Le Bonheur's Interventional Cardiac Imaging and Interventional Catheterization Laboratory, helped pioneer the transcatheter closure before joining an eight-site U.S. Food and Drug Administration (FDA)-approved trial for the Amplatzer Piccolo Occluder used in the closure. Le Bonheur enrolled more neonates than any other center in the study.
Shyam and his team closed the PDA in the first patient post-approval, a two-week-old baby girl, born at 28 weeks and weighing 1 kilogram.
Abbott's Piccolo device is smaller than a pea and now offers hope to premature infants and newborns who need corrective treatment, and who may be non-responsive to medical management and high risk to undergo corrective surgery.
Approximately 60,000 premature babies in the U.S. are born each year with a very low birth weight, and nearly 12,000 (one out of five) of these have a significant PDA which will require urgent treatment for the baby to survive.
For more information: www.abbott.com


 December 20, 2023
December 20, 2023