February 11, 2019 — Heart failure experts at the National Research Center for Cardiac Surgery in Astana, Kazakhstan, recently announced the first successful implantation of the FIVAD (Fully Implanted Ventricular Assist Device) into a human. An article about the implantation was also published in the Journal for Heart and Lung Transplantation.1
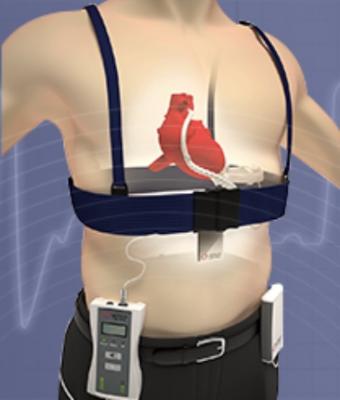
FIVAD is based on technology created by Leviticus Cardio, a medical technology company headquartered in Israel. It uses patented coplanar energy transfer (CET) to wirelessly power the heart pump, also known as a ventricular assist device (VAD). FIVAD incorporates a heart pump produced by Jarvik Heart Inc, an established manufacturer of ventricular assist devices.
Every year, VADs are surgically implanted into thousands of patients with severe heart failure in lieu of heart transplants. VADs need to continuously be connected to a power source, which requires patients to have a wire coming out of their bodies. This not only severely reduces the patients’ quality of life but, in over 20 percent of cases, causes infections which can lead to hospitalization and severe complications.
FIVAD is a fully implanted VAD system, a Jarvik 2000 pump, powered wirelessly using both internal and external components designed by Leviticus Cardi. This allows patients to walk around without any physical impediments for up to 8 hours a day.
After years of development and animal testing, the first implant of FIVAD in a human took place in December at the National Research Center for Cardiac Surgery in Astana. The patient has been discharged from the hospital and is back leading a normal life.
FIVAD is also equipped with a back-up system (Jarvik Heart, Post Auricular driveline connection) which would allow moving to traditional wired power in case the wireless system failed. While the backup was tested during the implant procedure, it has not been needed since that initial implant test.
Among the guests from the medical community participating in the press conference were:
-
Prof. Mandeep Mehra, professor of medicine, Harvard Medical School and editor-in-chief of The Journal of Heart and Lung Transplantation;
-
Prof. Nir Uriel, director, heart failure, transplant and mechanical circulatory support, University of Chicago and the charmian of the Mechanical Circulatory support council at the International Society of Heart Lung Transplantation;
-
Pya Yuriy Vladimirovich, M.D., CEO of National Research Center for Cardiac Surgery and the lead FIVAD surgeon;
-
Prof. Ivan Netuka, chairman of the Department of Cardiovascular Surgery, surgical director of chronic MCS and heart transplantation, Institute for Clinical and Experimental Medicine, Prague, who participated in the FIVAD surgery;
-
Prof. Stephan Schueler - consultant cardiothoracic surgeon at Newcastle upon Tyne Hospitals Trust and renowned cardiothoracic surgeon;
-
Assoc. Prof. Jiri Maly – deputy director/CMO of Institute for Clinical and Experimental Medicine, Prague, who participated in the FIVAD surgery; and
-
Yigal Kassif, M.D., attending cardiac surgeon and chief of cardiac surgery Intensive Care Unit at Sheba Medical Center in Israel.
Maly said, “We were really satisfied how easy it was to position the internal components of Leviticus’ system during surgery. It exceeded our expectations during the operation. Simplicity of surgery has definitely contributed to the patient’s early recovery”
Uriel added, “This is a significant improvement in the quality of life experienced by the patient. The patient has the freedom to go about his daily routine without having to worry about being connected to a power source via a driveline and can forget for a few hours that he is supported by an LVAD. We, the medical community, cardiologists, cardiac surgeons, VAD coordinators and the patients have wanted this for decades.”
For more information: www.leviticus-cardio.com, www.jarvikheart.com
Reference
1. Pya Y., Maly J., Bekbossynova M., et al. First Human Use of a Wireless Coplanar Energy Transfer Coupled with a Continuous-flow Left Ventricular Assist Device. The Journal of Heart and Lung Transplantation, Feb. 4, 2019.


 October 31, 2023
October 31, 2023 








