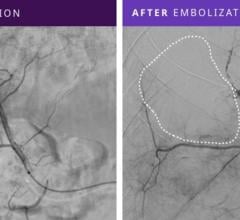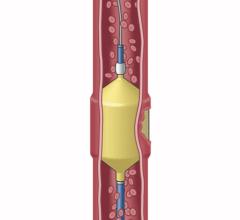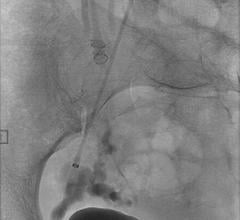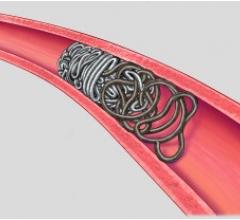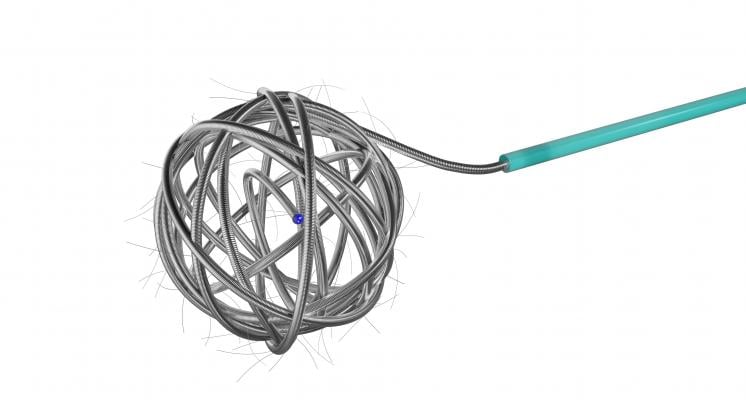
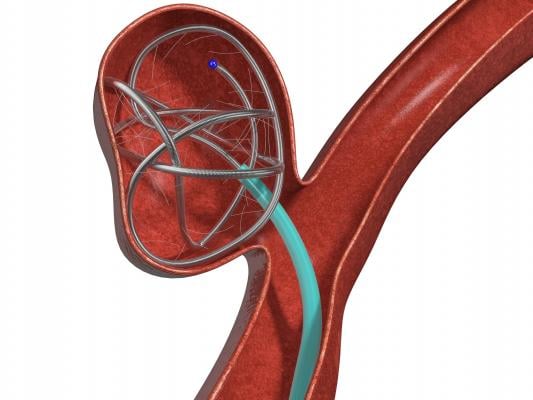
October 16, 2017 — Medtronic announced the launch of the Concerto 3-D Detachable Coil System at the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) meeting, Sept. 16-20 in Copenhagen, Denmark. The 3-D coil is an adjunctive product to the existing Concerto Helix line and is indicated for arterial and venous embolizations in the peripheral vasculature. The product is now available in both the United States and Europe.
The goal of arterial and venous embolization treatment is to prevent blood flow to a specific area. The new 3-D coil has a complex shape which gives physicians the ability to frame the treatment area by creating a scaffold. In addition, the coil also contains fibers that increase thrombogenicity (clotting).
For more information: www.medtronic.com

 July 28, 2022
July 28, 2022