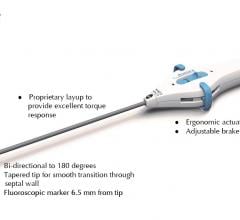
February 21, 2019 — Navitian, the new coronary microcatheter from iVascular, recently received CE mark approval. The device was approved to facilitate, guide and support a guidewire while accessing the coronary system, the exchange of guidewires, and injection of radiopaque contrast media or saline solutions.
Navitian was specifically designed to achieve a desirable navigation in cases of chronic total occlusions (CTOs). It stands out for its crossability and penetration capacity due to the internal and external conical transition.
Other key features of Navitian include:
-
Complete braided pattern that provides flexibility, pushability and resistance to kinking;
-
Hydrophilic coating Hydrax Plus having high trackability for small and tortuous arteries;
-
Internal polytetrafluoroethylene (PTFE) layer facilitating the movement of the guidewire during exchange; and
-
Sizes of 135 and 150 cm with guidewire compatibility of 0.014”.
For more information: www.ivascular.global


 April 03, 2024
April 03, 2024