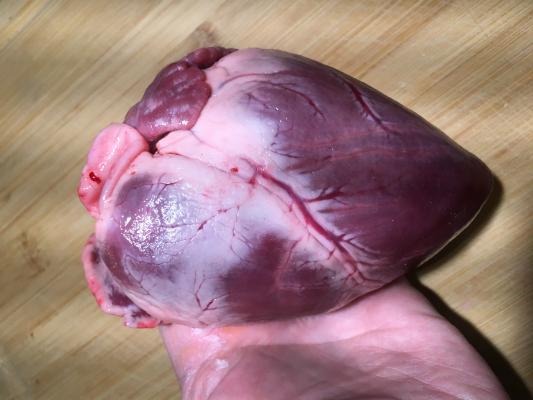
A pig heart, shown here, is very similar in size and anatomy to a human heart. For this reason, pigs are used extensively in pre-clinical animal testing for new implantable cardiovascular devices. If pig hearts could be used for human transplantation, it would greatly alleviate shortages of donor human hearts.
December 11, 2018 — The scientific journal Nature recently published an article from Munich University Hospital which describes the long-term survival of baboons that had received a heart transplant from genetically modified pigs.1 This is an important step forward on the way to being able to give humans porcine heart transplants.
Pig hearts are very similar in size, anatomy and function to human hearts, so are used to train medical students. Porcine hearts are the gold-standard in pre-clinical animal testing for all cardiovascular devices prior to use in humans to both test the safety and efficacy, and refine the implant procedures.
The article describes two requirements that have enabled the good results. One of these requirements is the introduction of non-ischemic heart preservation in accordance with the method using the products developed by Prof. Stig Steen and Swedish company XVIVO, and the other requirement is inhibition of post-transplantation growth of the heart, which otherwise would become too big for the primate.
XVIVO owns all commercial rights to the technology and is submitting an application to the Swedish Medical Products Agency as a prerequisite for a multicenter study on XVIVO’s products for heart preservation. The company plans to submit the application within approximately one month.
The XVIVO products consist of a preservation solution which has the same composition as that clinically used in the heart transplant study ongoing at the University Hospitals of Lund, earlier pre-clinical studies and now pre-clinically used in Munich for heart preservation in xenotransplantation. The technology also includes a portable heart preservation machine incorporating a single-use component which has been constructed by XVIVO, in accordance with Steen’s technology.
According to the United Network for Organ Sharing (UNOS), a total of 3,133 heart transplants have occurred in 2018, while 3,832 patients are currently waiting for a new heart,2 highlighting the need for creative solutions. An average of 20 people die every day while waiting for an organ transplant.2
For more information: www.xvivoperfusion.com
First Human Receives a Pig Heart Transplant using this technology (Jan. 7, 2022)
Reference
1. Längin M., Mayr T., Reichart B., et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature, published online Dec. 5, 2018. https://doi.org/10.1038/s41586-018-0765-z. Accessed Dec. 11, 2018.
2. https://unos.org/data/. Accessed Dec. 11, 2018.


 October 31, 2023
October 31, 2023 








