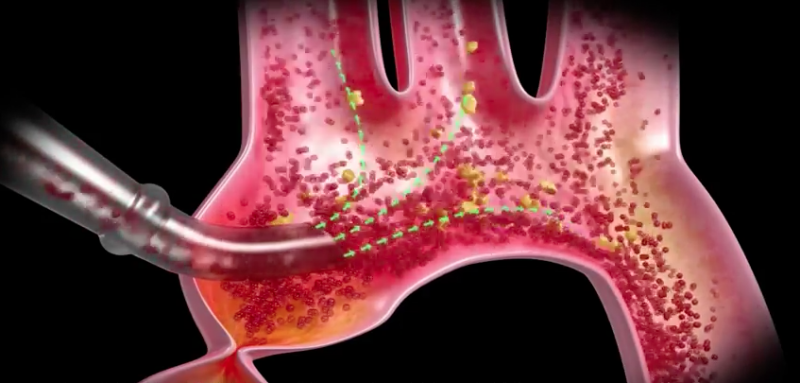
March 22, 2017 — Two U.S. Food and Drug Administration (FDA)-cleared embolic protection systems designed to remove ...
Aegis Medical Innovations Inc. announced that it has received Investigational Device Exemption approval from the U.S. Food and Drug Administration (FDA) to initiate a clinical trial in the U.S. for its Sierra Ligation System medical device. Aegis developed the Sierra technology in partnership with the Mayo Clinic in Rochester, Minn. Sierra could, with additional clinical research, prove to prevent stroke in patients with atrial fibrillation (AF), according to the company.

Here is a list of the American College of Cardiology (ACC) 2017 annual meeting late-breaking clinical trials presented ...
Providing exceptional cardiovascular care for patients to achieve the best possible outcomes is the number one goal for ...

March 22, 2017 – The U.S. Food and Drug Administration (FDA) and granted market clearance to the Medtronic CoreValve ...
March 21, 2017 — Toshiba Medical demonstrated its Forward projected model-based Iterative Reconstruction SoluTion (FIRST ...
March 21, 2017 — EchoPixel recently announced progress in the clinical adoption of its True 3D virtual reality software ...
Cardiac positron emission tomography (PET) is growing in popularity among cardiologists because it provides the ability ...
This video, provided by Abiomed, demonstrates the Impella percutaneous ventricular assist devices (pVAD). It offers ...

March 21, 2017 — About 12 percent of patients undergoing aortic valve replacement developed non-symptomatic blood clots ...
March 21, 2017 — A blood test for a protein called high-sensitivity troponin T, which is released into the bloodstream ...
When performing radiofrequency (RF) ablation to treat cardiac arrhythmia, medical professionals must balance the safety ...
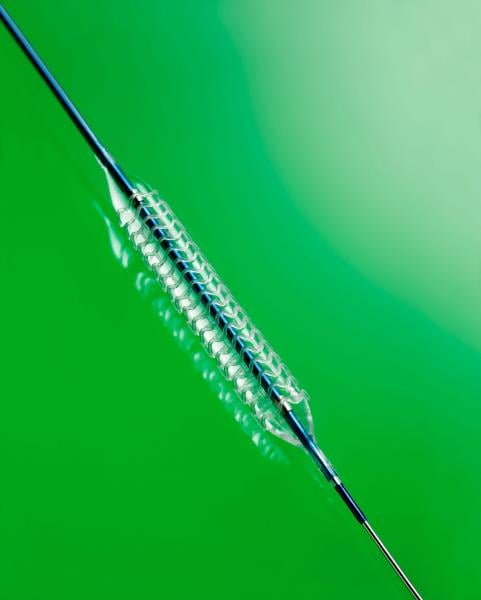
March 21, 2017 — In the late-breaking ABSORB III Trial two year results presented at the American College of Cardiology ...
March 20, 2017 — Teleflex Inc. recently announced 510(k) clearance by the U.S. Food and Drug Administration (FDA) and U ...
March 20, 2017 — Agfa HealthCare announced the release a new version of its Enterprise Imaging for Cardiology platform ...
Change Healthcare Cardiology Hemodynamics is an integrated hemodynamic monitoring system for monitoring vital signs and ...
March 20, 2017 — Cardiologists can now access the advanced ultrasound imaging technology needed for fast and confident ...

March 20, 2017 — For patients experiencing angina (chest pain) or a heart attack, instantaneous wave-free ratio (iFR) ...
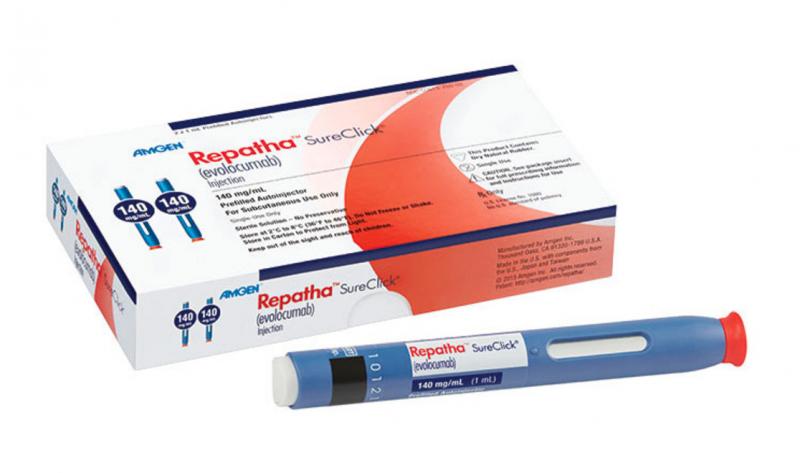
March 20, 2017 — The most important late-breaking pharmaceutical trial at the 2017 America College of Cardiology annual ...

 March 22, 2017
March 22, 2017








